Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis
- PMID: 15985551
- PMCID: PMC1175002
- DOI: 10.1073/pnas.0503891102
Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis
Abstract
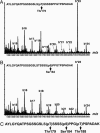
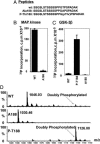
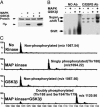
CCAAT enhancer-binding protein (C/EBP)beta, C/EBPalpha, and peroxisome proliferator activated receptor (PPAR)gamma act in a cascade where C/EBPbeta activates expression of C/EBPalpha and PPARgamma, which then function as pleiotropic activators of genes that produce the adipocyte phenotype. When growth-arrested 3T3-L1 preadipocytes are induced to differentiate, C/EBPbeta is rapidly expressed but still lacks DNA-binding activity. After a long (14-hour) lag, glycogen synthase kinase 3beta enters the nucleus, which correlates with hyperphosphorylation of C/EBPbeta and acquisition of DNA-binding activity. Concurrently, 3T3-L1 preadipocytes synchronously enter S phase and undergo mitotic clonal expansion, a prerequisite for terminal differentiation. Ex vivo and in vitro experiments with C/EBPbeta show that phosphorylation of Thr-188 by mitogen-activating protein kinase "primes" C/EBPbeta for subsequent phosphorylation on Ser-184 and Thr-179 by glycogen synthase kinase 3beta, acquisition of DNA-binding function, and transactivation of the C/EBPalpha and PPARgamma genes. The delayed transactivation of the C/EBPalpha and PPARgamma genes by C/EBPbeta appears necessary to allow mitotic clonal expansion, which would otherwise be prevented, because C/EBPalpha and PPARgamma are antimitotic.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

