Gemcitabine with or without continuous infusion 5-FU in advanced pancreatic cancer: a randomised phase II trial of the Italian oncology group for clinical research (GOIRC)
- PMID: 15986036
- PMCID: PMC2361554
- DOI: 10.1038/sj.bjc.6602640
Gemcitabine with or without continuous infusion 5-FU in advanced pancreatic cancer: a randomised phase II trial of the Italian oncology group for clinical research (GOIRC)
Abstract
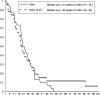
This study was performed to determine the activity of adding continuous infusion (CI) of 5-fluorouracil (5-FU) to gemcitabine (GEM) vs GEM alone in advanced pancreatic cancer (APC). In all, 94 chemo-naïve patients with APC were randomised to receive GEM alone (arm A: 1000 mg m(-2) per week for 7 weeks followed by a 2 week rest period, then weekly for 3 consecutive weeks out of every 4 weeks) or in combination with CI 5-FU (arm B: CI 5-FU 200 mg m(-2) day(-1) for 6 weeks followed by a 2 week rest period, then for 3 weeks every 4 weeks). Overall response rate (RR) was the primary end point and criteria for decision were planned according to the Simon's optimal two-stage design. The overall RR was 8% (arm A) and 11% (arm B) (95% confidence interval: 0.5-16% and 2-22%), respectively, and stable disease was 29 and 28%. The median duration of RR was 34 weeks (range 25-101 weeks) for GEM and 26 weeks (range 16-46 weeks) for the combination. The median progression-free survival (PFS) was 14 weeks (range 2-65 weeks) and 18 weeks (range 4-51 weeks), respectively. The median overall survival (OS) was 31 weeks (range 1-101 weeks) and 30 weeks (1-101 weeks). Toxicity was mild in both arms. This study does not show promising activity in terms of RR, PFS and OS for the double combination arm in APC.
Figures
References
-
- Alabiso O, Buosi R, Clerico M, Dogliotti L, Forti L, Lattuada S, Merlano M, Ostellino O, Satolli M, Alabiso I, Sponghini A (2001) Preliminary results of a phase II study with gemcitabine and continuous infusion 5-FU in patients with advanced or metastatic pancreatic cancer. Proc Am Soc Clin Oncol 20: A2331
-
- Anchisi S, Delaloye B, Petite J, Laurencet FL, Ambrod CH, Obrist R (2000) Gemcitabine (GEM) and continuous infusional 5-FU (Cif) is active and well tolerated in advanced or metastatic pancreatic cancer. Proc Am Soc Clin Oncol 19: A1280H
-
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson 3rd AB (2002) Phase III study of gemcitabine in combination with fluorouracil vs gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20: 3270–3275 - PubMed
-
- Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with GEM as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413 - PubMed
-
- Cartwright TH, Cohn A, Varkey JA, Chen YM, Szatrowski TP, Cox JV, Schulz JJ (2002) Phase II study of oral capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol 20: 160–164 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials