Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice
- PMID: 15986455
- PMCID: PMC1850986
- DOI: 10.1002/gene.20139
Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice
Abstract
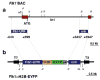
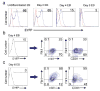
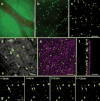
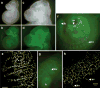
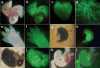
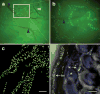
We report the first endothelial lineage-specific transgenic mouse allowing live imaging at subcellular resolution. We generated an H2B-EYFP fusion protein which can be used for fluorescent labeling of nucleosomes and used it to specifically label endothelial cells in mice and in differentiating embryonic stem (ES) cells. A fusion cDNA encoding a human histone H2B tagged at its C-terminus with enhanced yellow fluorescent protein (EYFP) was expressed under the control of an Flk1 promoter and intronic enhancer. The Flk1::H2B-EYFP transgenic mice are viable and high levels of chromatin-localized reporter expression are maintained in endothelial cells of developing embryos and in adult animals upon breeding. The onset of fluorescence in differentiating ES cells and in embryos corresponds with the beginning of endothelial cell specification. These transgenic lines permit real-time imaging in normal and pathological vasculogenesis and angiogenesis to track individual cells and mitotic events at a level of detail that is unprecedented in the mouse.
(c) 2005 Wiley-Liss, Inc.
Figures


References
-
- Anderson R, Copeland TK, Scholer H, Heasman J, Wylie C. The onset of germ cell migration in the mouse embryo. Mech Dev. 2000;91:61–68. - PubMed
-
- Bak M, Fraser SE. Axon fasciculation and differences in midline kinetics between pioneer and follower axons within commissural fascicles. Development. 2003;130:4999–5008. - PubMed
-
- Bautch VL, Ambler CA. Assembly and patterning of vertebrate blood vessels. Trends Cardiovasc Med. 2004;14:138–143. - PubMed
-
- Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med. 2001;7:864–868. - PubMed
-
- Caprioli A, Zhu H, Sato TN. CRBP-III:lacZ expression pattern reveals a novel heterogeneity of vascular endothelial cells. genesis. 2004;40:139–145. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

