Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497]
- PMID: 15987391
- PMCID: PMC1175879
- DOI: 10.1186/cc3495
Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497]
Abstract
Introduction: This randomised, open-label, multicentre study compared the safety and efficacy of an analgesia-based sedation regime using remifentanil with a conventional hypnotic-based sedation regime in critically ill patients requiring prolonged mechanical ventilation for up to 10 days.
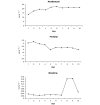
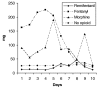
Methods: One hundred and five randomised patients received either a remifentanil-based sedation regime (initial dose 6 to 9 microg kg(-1) h(-1) (0.1 to 0.15 microg kg(-1) min(-1)) titrated to response before the addition of midazolam for further sedation (n = 57), or a midazolam-based sedation regime with fentanyl or morphine added for analgesia (n = 48). Patients were sedated to an optimal Sedation-Agitation Scale (SAS) score of 3 or 4 and a pain intensity (PI) score of 1 or 2.
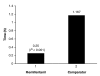
Results: The remifentanil-based sedation regime significantly reduced the duration of mechanical ventilation by more than 2 days (53.5 hours, P = 0.033), and significantly reduced the time from the start of the weaning process to extubation by more than 1 day (26.6 hours, P < 0.001). There was a trend towards shortening the stay in the intensive care unit (ICU) by 1 day. The median time of optimal SAS and PI was the same in both groups. There was a significant difference in the median time to offset of pharmacodynamic effects when discontinuing study medication in patients not extubated at 10 days (remifentanil 0.250 hour, comparator 1.167 hours; P < 0.001). Of the patients treated with remifentanil, 26% did not receive any midazolam during the study. In those patients that did receive midazolam, the use of remifentanil considerably reduced the total dose of midazolam required. Between days 3 and 10 the weighted mean infusion rate of remifentanil remained constant with no evidence of accumulation or of a development of tolerance to remifentanil. There was no difference between the groups in SAS or PI score in the 24 hours after stopping the study medication. Remifentanil was well tolerated.
Conclusion: Analgesia-based sedation with remifentanil was well tolerated; it reduces the duration of mechanical ventilation and improves the weaning process compared with standard hypnotic-based sedation regimes in ICU patients requiring long-term ventilation for up to 10 days.
Figures



Comment in
-
Narcotic-based sedation regimens for critically ill mechanically ventilated patients.Crit Care. 2005 Jun;9(3):247-8. doi: 10.1186/cc3523. Epub 2005 Apr 18. Crit Care. 2005. PMID: 15987412 Free PMC article.
References
-
- Egan TD, Lemmens HJ, Fiset P, Hermann DJ, Muir KT, Stanski DR, Shafer SL. The pharmacokinetics of the new short-acting opioid remifentanil (GI87084B) in healthy adult male volunteers. Anesthesiology. 1993;79:881–892. - PubMed
-
- Westmorland CL, Hoke JF, Sebel PS, Hugg CC, Jr, Muir KT. Pharmacokinetics of remifentanil (G187084B) and its major metabolite (GR90291) in patients undergoing elective surgery. Anesthesiology. 1993;79:893–903. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

