Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency
- PMID: 15987427
- PMCID: PMC1143555
- DOI: 10.1186/bcr1001
Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency
Abstract
Introduction: Dendritic cells (DCs) are antigen-presenting cells that are currently employed in cancer clinical trials. However, it is not clear whether their ability to induce tumour-specific immune responses when they are isolated from cancer patients is reduced relative to their ability in vivo. We determined the phenotype and functional activity of DCs from cancer patients and investigated the effect of putrescine, a polyamine molecule that is released in large amounts by cancer cells and has been implicated in metastatic invasion, on DCs.
Methods: The IL-4/GM-CSF (granulocyte-macrophage colony-stimulating factor) procedure for culturing blood monocyte-derived DCs was applied to cells from healthy donors and patients (17 with breast, 7 with colorectal and 10 with renal cell carcinoma). The same peroxide-treated tumour cells (M74 cell line) were used for DC pulsing. We investigated the effects of stimulation of autologous lymphocytes by DCs pulsed with treated tumour cells (DC-Tu), and cytolytic activity of T cells was determined in the same target cells.
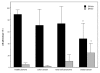
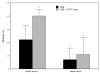
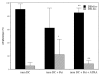
Results: Certain differences were observed between donors and breast cancer patients. The yield of DCs was dramatically weaker, and expression of MHC class II was lower and the percentage of HLA-DR-Lin- cells higher in patients. Whatever combination of maturating agents was used, expression of markers of mature DCs was significantly lower in patients. Also, DCs from patients exhibited reduced ability to stimulate cytotoxic T lymphocytes. After DC-Tu stimulation, specific cytolytic activity was enhanced by up to 40% when DCs were from donors but only up to 10% when they were from patients. IFN-gamma production was repeatedly found to be enhanced in donors but not in patients. By adding putrescine to DCs from donors, it was possible to enhance the HLA-DR-Lin- cell percentage and to reduce the final cytolytic activity of lymphocytes after DC-Tu stimulation, mimicking defective DC function. These putrescine-induced deficiencies were reversed by treating DCs with all-trans retinoic acid.
Conclusion: These data are consistent with blockade of antigen-presenting cells at an early stage of differentiation in patients with breast cancer. Putrescine released in the microenvironmement of DCs could be involved in this blockade. Use of all-trans retinoic acid treatment to reverse this blockade and favour ex vivo expansion of antigen-specific T lymphocytes is of real interest.
Figures


Similar articles
-
Induction of G250-targeted and T-cell-mediated antitumor activity against renal cell carcinoma using a chimeric fusion protein consisting of G250 and granulocyte/monocyte-colony stimulating factor.Cancer Res. 2001 Nov 1;61(21):7925-33. Cancer Res. 2001. PMID: 11691814
-
Presentation of renal tumor antigens by human dendritic cells activates tumor-infiltrating lymphocytes against autologous tumor: implications for live kidney cancer vaccines.Clin Cancer Res. 1999 Feb;5(2):445-54. Clin Cancer Res. 1999. PMID: 10037196
-
Autologous dendritic cells loaded with apoptotic tumor cells induce T cell-mediated immune responses against breast cancer in vitro.Cell Immunol. 2009;257(1-2):23-31. doi: 10.1016/j.cellimm.2009.02.002. Cell Immunol. 2009. PMID: 19306994
-
Dendritic cell-tumor fusion vaccines for renal cell carcinoma.Clin Cancer Res. 2004 Sep 15;10(18 Pt 2):6347S-52S. doi: 10.1158/1078-0432.CCR-050005. Clin Cancer Res. 2004. PMID: 15448029 Review.
-
Dendritic cells (II): Role and therapeutic implications in cancer.J R Coll Surg Edinb. 2001 Jun;46(3):159-67. J R Coll Surg Edinb. 2001. PMID: 11478013 Review.
Cited by
-
Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer.Cancer Immunol Immunother. 2013 May;62(5):909-18. doi: 10.1007/s00262-013-1396-8. Epub 2013 Apr 16. Cancer Immunol Immunother. 2013. PMID: 23589106 Free PMC article. Clinical Trial.
-
Engineering an active immunotherapy for personalized cancer treatment and prevention of recurrence.Sci Adv. 2023 Apr 28;9(17):eade0625. doi: 10.1126/sciadv.ade0625. Epub 2023 Apr 26. Sci Adv. 2023. PMID: 37126558 Free PMC article.
-
Interrogating Cellular Functions with Designer Janus Particles.Chem Mater. 2017 Feb 28;29:1448-1460. doi: 10.1021/acs.chemmater.6b05322. Epub 2017 Jan 20. Chem Mater. 2017. PMID: 31530969 Free PMC article.
-
Ex vivo development, expansion and in vivo analysis of a novel lineage of dendritic cells from hematopoietic stem cells.J Immune Based Ther Vaccines. 2010 Nov 24;8:8. doi: 10.1186/1476-8518-8-8. J Immune Based Ther Vaccines. 2010. PMID: 21106069 Free PMC article.
-
Optimizing DC vaccination by combination with oncolytic adenovirus coexpressing IL-12 and GM-CSF.Mol Ther. 2011 Aug;19(8):1558-68. doi: 10.1038/mt.2011.29. Epub 2011 Apr 5. Mol Ther. 2011. PMID: 21468000 Free PMC article.
References
-
- Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–433. doi: 10.1084/jem.191.3.423. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

