Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies
- PMID: 15987448
- PMCID: PMC1175054
- DOI: 10.1186/bcr1020
Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies
Abstract
Introduction: The purpose of this retrospective study was to determine the clinical utility of serum HER2/neu in monitoring metastatic breast cancer patients undergoing trastuzumab-based therapy and to compare these results with those obtained using cancer antigen (CA) 15-3. We also sought to determine whether early changes in serum HER2/neu concentrations could be a predictor of progression-free survival.
Methods: Sera were obtained retrospectively from 103 women at four medical institutions. Patients eligible for participation were women with metastatic breast cancer who had HER2/neu tissue overexpression and were scheduled to be treated with trastuzumab with or without additional therapies as per the established practices of the treating physicians. A baseline serum sample for each patient was taken before trastuzumab-based therapy was started. Patients were subsequently monitored over 12 to 20 months and serum samples were taken at the time of clinical assessment and tested with Bayer's HER2/neu and CA15-3 assays.
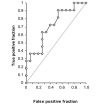
Results: Concordance between clinical status in patients undergoing trastuzumab-based treatment and HER2/neu and CA15-3 used as single tests was 0.793 and 0.627, respectively, and increased to 0.829 when the tests were used in combination. Progression-free survival times did not differ significantly in patients with elevated baseline HER2/neu concentrations (> or = 15 ng/mL) and those with normal concentrations (<15 ng/mL). However, progression-free survival differed significantly (P = 0.043) according to whether the patient's HER2/neu concentration at 2 to 4 weeks after the start of therapy was >77% or < or = 77% of her baseline concentration. The median progression-free survival times for these two groups were 217 and 587 days, respectively. A similar trend was observed for a subcohort of patients treated specifically with a combination of trastuzumab and taxane.
Conclusion: These findings indicate that serum HER2/neu testing is clinically valuable in monitoring metastatic breast cancer patients undergoing trastuzumab-based treatment and provides additional value over the commonly used CA15-3 test. The percentage of baseline HER2/neu concentrations in the early weeks after the start of therapy may be an early predictor of progression-free-survival.
Figures


References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, Slamon DJ. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000;18:3651–3664. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
-
- Elledge RM, Green S, Ciocca D, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, O'Sullivan J, Martino S, et al. HER-2 expression and response to tamoxifen in estrogen receptor-positive breast cancer: a Southwest Oncology Group Study. Clin Cancer Res. 1998;4:7–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

