Mammary epithelial cell transformation: insights from cell culture and mouse models
- PMID: 15987472
- PMCID: PMC1175079
- DOI: 10.1186/bcr1275
Mammary epithelial cell transformation: insights from cell culture and mouse models
Abstract
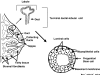
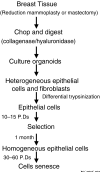
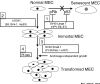
Normal human mammary epithelial cells (HMECs) have a finite life span and do not undergo spontaneous immortalization in culture. Critical to oncogenic transformation is the ability of cells to overcome the senescence checkpoints that define their replicative life span and to multiply indefinitely -- a phenomenon referred to as immortalization. HMECs can be immortalized by exposing them to chemicals or radiation, or by causing them to overexpress certain cellular genes or viral oncogenes. However, the most efficient and reproducible model of HMEC immortalization remains expression of high-risk human papillomavirus (HPV) oncogenes E6 and E7. Cell culture models have defined the role of tumor suppressor proteins (pRb and p53), inhibitors of cyclin-dependent kinases (p16INK4a, p21, p27 and p57), p14ARF, telomerase, and small G proteins Rap, Rho and Ras in immortalization and transformation of HMECs. These cell culture models have also provided evidence that multiple epithelial cell subtypes with distinct patterns of susceptibility to oncogenesis exist in the normal mammary tissue. Coupled with information from distinct molecular portraits of primary breast cancers, these findings suggest that various subtypes of mammary cells may be precursors of different subtypes of breast cancers. Full oncogenic transformation of HMECs in culture requires the expression of multiple gene products, such as SV40 large T and small t, hTERT (catalytic subunit of human telomerase), Raf, phosphatidylinositol 3-kinase, and Ral-GEFs (Ral guanine nucleotide exchange factors). However, when implanted into nude mice these transformed cells typically produce poorly differentiated carcinomas and not adenocarcinomas. On the other hand, transgenic mouse models using ErbB2/neu, Ras, Myc, SV40 T or polyomavirus T develop adenocarcinomas, raising the possibility that the parental normal cell subtype may determine the pathological type of breast tumors. Availability of three-dimensional and mammosphere models has led to the identification of putative stem cells, but more studies are needed to define their biologic role and potential as precursor cells for distinct breast cancers. The combined use of transformation strategies in cell culture and mouse models together with molecular definition of human breast cancer subtypes should help to elucidate the nature of breast cancer diversity and to develop individualized therapies.
Figures

References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Taylor-Papadimitriou J, Berdichevsky F, D'Souza B, Burchell J. Human models of breast cancer. Cancer Surv. 1993;16:59–78. - PubMed
-
- Farber E. The multistep nature of cancer development. Cancer Res. 1984;44:4217–4223. - PubMed
-
- Ratsch SB, Gao Q, Srinivasan S, Wazer DE, Band V. Multiple genetic changes are required for efficient immortalization of different subtypes of normal human mammary epithelial cells. Radiat Res. 2001;155:143–150. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA087986/CA/NCI NIH HHS/United States
- R01 CA096844/CA/NCI NIH HHS/United States
- CA96844/CA/NCI NIH HHS/United States
- CA81076/CA/NCI NIH HHS/United States
- R01 CA094143/CA/NCI NIH HHS/United States
- CA 87986/CA/NCI NIH HHS/United States
- CA94143/CA/NCI NIH HHS/United States
- CA 76118/CA/NCI NIH HHS/United States
- CA99163/CA/NCI NIH HHS/United States
- CA 99900/CA/NCI NIH HHS/United States
- R01 CA099900/CA/NCI NIH HHS/United States
- R01 CA081076/CA/NCI NIH HHS/United States
- R01 CA099163/CA/NCI NIH HHS/United States
- CA 094150/CA/NCI NIH HHS/United States
- R01 CA094150/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

