The impact of a pharmacist-managed dosage form conversion service on ciprofloxacin usage at a major Canadian teaching hospital: a pre- and post-intervention study
- PMID: 15987523
- PMCID: PMC1185535
- DOI: 10.1186/1472-6963-5-48
The impact of a pharmacist-managed dosage form conversion service on ciprofloxacin usage at a major Canadian teaching hospital: a pre- and post-intervention study
Abstract
Background: Despite cost containment efforts, parenteral (IV) ciprofloxacin appears to be overutilized at Vancouver General Hospital. In November 2003, the Pharmacist-managed intravenous to oral (IV-PO) Dosage Form Conversion Service was implemented, enabling autonomous pharmacist-initiated dosage form conversion for ciprofloxacin. This study evaluates characteristics of ciprofloxacin use prior to and following implementation of this conversion service.
Methods: This was a single-centre, two-phase (pre/post), unblinded study. Phase I occurred between November 12, 2002 and November 11, 2003 (365 days), and Phase II between November 12, 2003 and March 11, 2004 (120 days). All patients receiving ciprofloxacin IV during these periods were reviewed. The primary endpoint was IV:PO ciprofloxacin use ratio. Secondary endpoints were total number of ciprofloxacin doses, proportion of inappropriate IV ciprofloxacin doses, cost of therapy between phases, and estimated cost avoidance with the intervention.
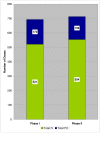
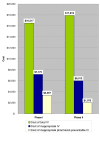
Results: Two hundred ciprofloxacin IV treatment courses were evaluated (100 per phase). The IV:PO ciprofloxacin use ratio was 3.03 (Phase I) vs. 3.48 (Phase II). Total number of doses and ratio of IV to total doses across phases were similar (p = 0.2830). IV-PO ciprofloxacin conversion occurred in 27/100 (27%) of IV courses in Phase I and 23/100 (23%) in Phase II. Proportion of inappropriate ciprofloxacin IV doses decreased between Phases I and II (244/521 (47%) vs. 201/554 (36%) (p = 0.0005), respectively). Furthermore, the proportion of pharmacist-preventable inappropriate ciprofloxacin IV doses was reduced between Phases I and II (114/244 (47%) vs. 65/201 (32%) (p = 0.0026). Proportional cost avoidance associated with total inappropriate IV use was 7,172 Can dollars/16,517 Can dollars (43%) (in Canadian dollars) in Phase I vs. 6,012 Can dollars/17,919 Can dollars (34%) in Phase II (p = 0.001). Similarly, proportional cost avoidance associated with pharmacist-preventable inappropriate IV doses was reduced from 3,367 Can dollars/16,517 Can dollars (20%) in Phase I to 1,975 Can dollars/17,919 Can dollars (11%) in Phase II (p = 0.001).
Conclusion: While overall utilization of ciprofloxacin remained unchanged and the proportion of IV to total doses was stable during the study period, the proportion of inappropriate IV doses and its associated costs appear to have declined subsequent to implementation of a Pharmacist-managed IV-PO Dosage Form Conversion Service. Such a program may be a beneficial adjunct in facilitating appropriate and cost-effective usage of ciprofloxacin.
Figures

References
-
- Bayer Inc . Cipro® product monograph. In: Repchinsky C, editor. Compendium of pharmaceuticals and specialties 2004: the Canadian drug reference for health professionals. Ottawa, ON Canadian Pharmacists Association; 2004. pp. 425–428.
-
- Echols RM. Antimicrobial practice. The selection of appropriate dosages for intravenous ciprofloxacin. J Antimicrob Chemother. 1993;31:783–787. - PubMed
-
- Drugs and Therapeutics Committee . In: Formulary of Vancouver Hospital and Health Sciences Centre. Shalansky K, Hill S. Vancouver, BC, editor. Vancouver Hospital and Health Sciences Centre; 2003. p. 14.
-
- Frighetto L, Martinusen SM, Mamdani F, Jewesson PJ. Ciprofloxacin use under a reserved drug and stepdown promotion program. Can J Hosp Pharm. 1995;48:35–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

