Silencing the epidermal growth factor receptor gene with RNAi may be developed as a potential therapy for non small cell lung cancer
- PMID: 15987532
- PMCID: PMC1187910
- DOI: 10.1186/1479-0556-3-5
Silencing the epidermal growth factor receptor gene with RNAi may be developed as a potential therapy for non small cell lung cancer
Abstract
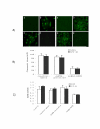
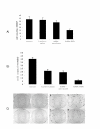
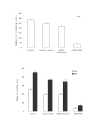
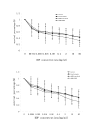
Lung cancer has emerged as a leading cause of cancer death in the world. Non-small cell lung cancer (NSCLC) accounts for 75-80% of all lung cancers. Current therapies are ineffective, thus new approaches are needed to improve the therapeutic ratio. Double stranded RNA (dsRNA)-mediated RNA interference (RNAi) has shown promise in gene silencing, the potential of which in developing new methods for the therapy of NSCLC needs to be tested. We report here RNAi induced effective silencing of the epidermal growth factor receptor (EGFR) gene, which is over expressed in NSCLC. NSCLC cell lines A549 and SPC-A1 were transfected with sequence- specific dsRNA as well as various controls. Immune fluorescent labeling and flow cytometry were used to monitor the reduction in the production of EGFR protein. Quantitative reverse-transcriptase PCR was used to detect the level of EGFR mRNA. Cell count, colony assay, scratch assay, MTT assay in vitro and tumor growth assay in athymic nude mice in vivo were used to assess the functional effects of EGFR silencing on tumor cell growth and proliferation. Our data showed transfection of NSCLC cells with dsRNA resulted in sequence specific silencing of EGFR with 71.31% and 71.78 % decreases in EGFR protein production and 37.04% and 54.92% in mRNA transcription in A549 and SPC-A1 cells respectively. The decrease in EGFR protein production caused significant growth inhibition, i.e.: reducing the total cell numbers by 85.0% and 78.3%, and colony forming numbers by 63.3% and 66.8%. These effects greatly retarded the migration of NSCLC cells by more than 80% both at 24 h and at 48 h, and enhanced chemo-sensitivity to cisplatin by four-fold in A549 cells and seven-fold in SPC-A1. Furthermore, dsRNA specific for EGFR inhibited tumor growth in vivo both in size by 75.06% and in weight by 73.08%. Our data demonstrate a new therapeutic effect of sequence specific suppression of EGFR gene expression by RNAi, enabling inhibition of tumor proliferation and growth. However, in vivo use of dsRNA for gene transfer to tumor cells would be limited because dsRNA would be quickly degraded once delivered in vivo. We thus tested a new bovine lentiviral vector and showed lentivector-mediated RNAi effects were efficient and specific. Combining RNAi with this gene delivery system may enable us to develop RNAi for silencing EGFR into an effective therapy for NSCLC.
Figures

Similar articles
-
[Antitumor effect of epidermal growth factor receptor gene silencing].Zhonghua Jie He He Hu Xi Za Zhi. 2005 May;28(5):337-41. Zhonghua Jie He He Hu Xi Za Zhi. 2005. PMID: 15949316 Chinese.
-
[Antitumor effect of RNA interference on non-small cell lung cancer in vivo].Zhonghua Zhong Liu Za Zhi. 2008 Nov;30(11):804-7. Zhonghua Zhong Liu Za Zhi. 2008. PMID: 19173822 Chinese.
-
[Effects of RNA interference on epidermal growth factor receptor expression in SPC-A-1 cells].Zhonghua Nei Ke Za Zhi. 2004 May;43(5):345-8. Zhonghua Nei Ke Za Zhi. 2004. PMID: 15182504 Chinese.
-
[Relationship between epidermal growth factor receptor gene expression and radiosensitivity of non-small-cell lung cancer cells].Zhonghua Zhong Liu Za Zhi. 2013 Feb;35(2):94-7. doi: 10.3760/cma.j.issn.0253-3766.2013.02.004. Zhonghua Zhong Liu Za Zhi. 2013. PMID: 23714661 Chinese.
-
Small RNA: can RNA interference be exploited for therapy?Lancet. 2003 Oct 25;362(9393):1401-3. doi: 10.1016/S0140-6736(03)14637-5. Lancet. 2003. PMID: 14585643 Review.
Cited by
-
Enhancing chemosensitivity in oral squamous cell carcinoma by lentivirus vector-mediated RNA interference targeting EGFR and MRP2.Oncol Lett. 2016 Sep;12(3):2107-2114. doi: 10.3892/ol.2016.4883. Epub 2016 Jul 20. Oncol Lett. 2016. PMID: 27602148 Free PMC article.
-
Antitumor activity of a novel EGFR tyrosine kinase inhibitor against human lung carcinoma in vitro and in vivo.Invest New Drugs. 2009 Feb;27(1):1-11. doi: 10.1007/s10637-008-9132-5. Epub 2008 May 21. Invest New Drugs. 2009. PMID: 18493719
-
Non-coding RNAs in lung cancer.Oncoscience. 2014 Nov 15;1(11):674-705. doi: 10.18632/oncoscience.98. eCollection 2014. Oncoscience. 2014. PMID: 25593996 Free PMC article. Review.
-
Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity.J Biol Chem. 2010 Aug 6;285(32):24775-82. doi: 10.1074/jbc.M110.134585. Epub 2010 Jun 10. J Biol Chem. 2010. PMID: 20538600 Free PMC article.
-
Localized RNA interference therapy to eliminate residual lung cancer after incomplete microwave ablation.Thorac Cancer. 2019 Jun;10(6):1369-1377. doi: 10.1111/1759-7714.13079. Epub 2019 Apr 24. Thorac Cancer. 2019. PMID: 31017731 Free PMC article.
References
-
- McLennan G., Roder DM. Lung cancer in Australia. Med J Aust. 1989;150:206–207. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

