Multiple myosin II heavy chain kinases: roles in filament assembly control and proper cytokinesis in Dictyostelium
- PMID: 15987738
- PMCID: PMC1196335
- DOI: 10.1091/mbc.e05-03-0219
Multiple myosin II heavy chain kinases: roles in filament assembly control and proper cytokinesis in Dictyostelium
Abstract
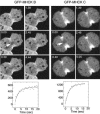
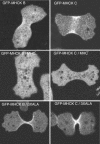
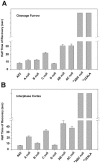
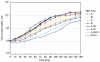
Myosin II filament assembly in Dictyostelium discoideum is regulated via phosphorylation of residues located in the carboxyl-terminal portion of the myosin II heavy chain (MHC) tail. A series of novel protein kinases in this system are capable of phosphorylating these residues in vitro, driving filament disassembly. Previous studies have demonstrated that at least three of these kinases (MHCK A, MHCK B, and MHCK C) display differential localization patterns in living cells. We have created a collection of single, double, and triple gene knockout cell lines for this family of kinases. Analysis of these lines reveals that three MHC kinases appear to represent the majority of cellular activity capable of driving myosin II filament disassembly, and reveals that cytokinesis defects increase with the number of kinases disrupted. Using biochemical fractionation of cytoskeletons and in vivo measurements via fluorescence recovery after photobleaching (FRAP), we find that myosin II overassembly increases incrementally in the mutants, with the MHCK A(-)/B(-)/C(-) triple mutant showing severe myosin II overassembly. These studies suggest that the full complement of MHC kinases that significantly contribute to growth phase and cytokinesis myosin II disassembly in this organism has now been identified.
Figures





References
-
- Abu-Elneel, K., Karchi, M., and Ravid, S. (1996). Dictyostelium myosin II is regulated during chemotaxis by a novel protein kinase C (PKC). J. Biol. Chem. 271, 977-984. - PubMed
-
- Baumann, O. (2004). Spatial pattern of nonmuscle myosin-II distribution during the development of the Drosophila compound eye and implications for retinal morphogenesis. Dev. Biol. 269, 519-533. - PubMed
-
- Betapudi, V., Shoebotham, K., and Egelhoff, T. T. (2004). Generation of double gene disruptions in Dictyostelium discoideum using a single antibiotic marker selection. Biotechniques 36, 106-112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

