EID3 is a novel EID family member and an inhibitor of CBP-dependent co-activation
- PMID: 15987788
- PMCID: PMC1159117
- DOI: 10.1093/nar/gki667
EID3 is a novel EID family member and an inhibitor of CBP-dependent co-activation
Abstract
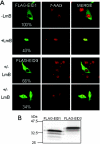
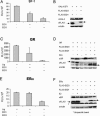
EID1 (E1A-like inhibitor of differentiation 1) functions as an inhibitor of nuclear receptor-dependent gene transcription by directly binding to co-regulators. Alternative targets include the co-repressor small heterodimer partner (SHP, NR0B2) and the co-activators CBP/p300, indicating that EID1 utilizes different inhibitory strategies. Recently, EID2 was characterized as an inhibitor of muscle differentiation and as an antagonist of both CBP/p300 and HDACs. Here, we describe a third family member designated EID3 that is highly expressed in testis and shows homology to a region of EID1 implicated in binding to CBP/p300. We demonstrate that EID3 acts as a potent inhibitor of nuclear receptor transcriptional activity by a mechanism that is independent of direct interactions with nuclear receptors, including SHP. Furthermore, EID3 directly binds to and blocks the SRC-1 interacting domain of CBP, which has been implicated to act as the interaction surface for nuclear receptor co-activators. Consistent with this idea, EID3 prevents recruitment of CBP to a natural nuclear receptor-regulated promoter. Our study suggests that EID-family members EID3 and EID1 act as inhibitors of CBP/p300-dependent transcription in a tissue-specific manner.
Figures




Similar articles
-
Cells degrade a novel inhibitor of differentiation with E1A-like properties upon exiting the cell cycle.Mol Cell Biol. 2000 Dec;20(23):8889-902. doi: 10.1128/MCB.20.23.8889-8902.2000. Mol Cell Biol. 2000. PMID: 11073989 Free PMC article.
-
Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP.Nature. 1995 Mar 2;374(6517):85-8. doi: 10.1038/374085a0. Nature. 1995. PMID: 7870179
-
A transcriptional inhibitor targeted by the atypical orphan nuclear receptor SHP.EMBO Rep. 2002 May;3(5):478-84. doi: 10.1093/embo-reports/kvf087. Epub 2002 Apr 18. EMBO Rep. 2002. PMID: 11964378 Free PMC article.
-
Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action.Recent Prog Horm Res. 1997;52:141-64; discussion 164-5. Recent Prog Horm Res. 1997. PMID: 9238851 Review.
-
Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state.Nat Rev Cancer. 2007 Sep;7(9):713-22. doi: 10.1038/nrc2211. Nat Rev Cancer. 2007. PMID: 17721435 Review.
Cited by
-
Truncating SRCAP variants outside the Floating-Harbor syndrome locus cause a distinct neurodevelopmental disorder with a specific DNA methylation signature.Am J Hum Genet. 2021 Jun 3;108(6):1053-1068. doi: 10.1016/j.ajhg.2021.04.008. Epub 2021 Apr 27. Am J Hum Genet. 2021. PMID: 33909990 Free PMC article.
-
Identification of the proteins, including MAGEG1, that make up the human SMC5-6 protein complex.Mol Cell Biol. 2008 Feb;28(4):1197-206. doi: 10.1128/MCB.00767-07. Epub 2007 Dec 17. Mol Cell Biol. 2008. PMID: 18086888 Free PMC article.
-
Analysis of the Nse3/MAGE-binding domain of the Nse4/EID family proteins.PLoS One. 2012;7(4):e35813. doi: 10.1371/journal.pone.0035813. Epub 2012 Apr 20. PLoS One. 2012. PMID: 22536443 Free PMC article.
-
DNA methylome profiling identifies novel methylated genes in African American patients with colorectal neoplasia.Epigenetics. 2014 Apr;9(4):503-12. doi: 10.4161/epi.27644. Epub 2014 Jan 17. Epigenetics. 2014. PMID: 24441198 Free PMC article.
-
Grainyhead-like 2 inhibits the coactivator p300, suppressing tubulogenesis and the epithelial-mesenchymal transition.Mol Biol Cell. 2016 Aug 1;27(15):2479-92. doi: 10.1091/mbc.E16-04-0249. Epub 2016 Jun 1. Mol Biol Cell. 2016. PMID: 27251061 Free PMC article.
References
-
- Eckner R., Ewen M., Newsome D., Gerdes M., DeCaprio J., Lawrence J., Livingston D. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. - PubMed
-
- Goodman R.H., Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. - PubMed
-
- Sang N., Caro J., Giordano A. Adenoviral E1A: everlasting tool, versatile applications, continuous contributions and new hypotheses. Front. Biosci. 2002;7:d407–413. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

