Identifying Plasmodium falciparum merozoite surface antigen 3 (MSP3) protein peptides that bind specifically to erythrocytes and inhibit merozoite invasion
- PMID: 15987906
- PMCID: PMC2253348
- DOI: 10.1110/ps.041304505
Identifying Plasmodium falciparum merozoite surface antigen 3 (MSP3) protein peptides that bind specifically to erythrocytes and inhibit merozoite invasion
Abstract
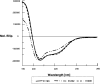
Receptor-ligand interactions between synthetic peptides and normal human erythrocytes were studied to determine Plasmodium falciparum merozoite surface protein-3 (MSP-3) FC27 strain regions that specifically bind to membrane surface receptors on human erythrocytes. Three MSP-3 protein high activity binding peptides (HABPs) were identified; their binding to erythrocytes became saturable, had nanomolar affinity constants, and became sensitive on being treated with neuraminidase and trypsin but were resistant to chymotrypsin treatment. All of them specifically recognized 45-, 55-, and 72-kDa erythrocyte membrane proteins. They all presented alpha-helix structural elements. All HABPs inhibited in vitro P. falciparum merozoite invasion of erythrocytes by ~55%-85%, suggesting that MSP-3 protein's role in the invasion process probably functions by using mechanisms similar to those described for other MSP family antigens.
Figures


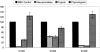

Similar articles
-
Identifying Plasmodium falciparum merozoite surface protein-10 human erythrocyte specific binding regions.Biochimie. 2005 May;87(5):461-72. doi: 10.1016/j.biochi.2005.01.001. Biochimie. 2005. PMID: 15820753
-
Plasmodium falciparum merozoite surface protein 6 (MSP-6) derived peptides bind erythrocytes and partially inhibit parasite invasion.Peptides. 2006 Jul;27(7):1685-92. doi: 10.1016/j.peptides.2006.02.001. Epub 2006 May 19. Peptides. 2006. PMID: 16713025
-
Identifying merozoite surface protein 4 and merozoite surface protein 7 Plasmodium falciparum protein family members specifically binding to human erythrocytes suggests a new malarial parasite-redundant survival mechanism.J Med Chem. 2007 Nov 15;50(23):5665-75. doi: 10.1021/jm070773z. Epub 2007 Oct 18. J Med Chem. 2007. PMID: 17944453
-
Emerging rules for subunit-based, multiantigenic, multistage chemically synthesized vaccines.Acc Chem Res. 2008 Mar;41(3):377-86. doi: 10.1021/ar700120t. Epub 2008 Feb 12. Acc Chem Res. 2008. PMID: 18266328 Review.
-
Invasion of host cells by malaria parasites: a tale of two protein families.Mol Microbiol. 2007 Jul;65(2):231-49. doi: 10.1111/j.1365-2958.2007.05791.x. Mol Microbiol. 2007. PMID: 17630968 Review.
Cited by
-
The C-terminal domain of Plasmodium falciparum merozoite surface protein 3 self-assembles into alpha-helical coiled coil tetramer.Mol Biochem Parasitol. 2009 Jun;165(2):153-61. doi: 10.1016/j.molbiopara.2009.01.015. Epub 2009 Feb 10. Mol Biochem Parasitol. 2009. PMID: 19428662 Free PMC article.
-
Plasmodium falciparum MSP3 Exists in a Complex on the Merozoite Surface and Generates Antibody Response during Natural Infection.Infect Immun. 2018 Jul 23;86(8):e00067-18. doi: 10.1128/IAI.00067-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29760216 Free PMC article.
-
Plasmodium falciparum merozoite surface protein 3: oligomerization, self-assembly, and heme complex formation.J Biol Chem. 2014 Feb 14;289(7):3856-68. doi: 10.1074/jbc.M113.520239. Epub 2013 Dec 19. J Biol Chem. 2014. PMID: 24362023 Free PMC article.
-
Genetic diversity of the malaria vaccine candidate Plasmodium falciparum merozoite surface protein-3 in a hypoendemic transmission environment.Am J Trop Med Hyg. 2009 Mar;80(3):479-86. Am J Trop Med Hyg. 2009. PMID: 19270302 Free PMC article.
-
Genetic polymorphism of merozoite surface protein-3 in Myanmar Plasmodium falciparum field isolates.Malar J. 2020 May 19;19(1):184. doi: 10.1186/s12936-020-03256-y. Malar J. 2020. PMID: 32429986 Free PMC article.
References
-
- Adler, A.J., Greenfield, N.J., and Fasman, G.D. 1973. Circular dichroism and optical rotatory dispersion of proteins and polypeptides. Methods Enzymol. 27 675–735. - PubMed
-
- Black, C.G., Wu, T., Wang, L., Hibbs, A.R., and Coppel, R.L. 2001. Merozoite surface protein 8 of Plasmodium falciparum contains two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 114 217–226. - PubMed
-
- Carvalho, L.J., Oliveira, S.G., Theisen, M., Alves, F.A., Andrade, M.C., Zanini, G.M., Brigido, M.C., Oeuvray, C., Povoa, M.M., Muniz, J.A., et al. 2004. Immunization of Saimiri sciureus monkeys with Plasmodium falciparum merozoite surface protein-3 and glutamate-rich protein suggests that protection is related to antibody levels. Scand. J. Immunol. 59 363–372. - PubMed
-
- Curtidor, H., Urquiza, M., Suarez, J.E., Rodriguez, L.E., Ocampo, M., Puentes, A., Garcia, J.E., Vera, R., Lopez, R., Ramirez, L.E., et al. 2001. Plasmodium falciparum acid basic repeat antigen (ABRA) peptides: Erythrocyte binding and biological activity. Vaccine 19 4496–4504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

