Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network
- PMID: 15987953
- PMCID: PMC6725057
- DOI: 10.1523/JNEUROSCI.1478-05.2005
Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network
Abstract
Behavioral conditioning of cue-reward pairing results in a shift of midbrain dopamine (DA) cell activity from responding to the reward to responding to the predictive cue. However, the precise time course and mechanism underlying this shift remain unclear. Here, we report a combined single-unit recording and temporal difference (TD) modeling approach to this question. The data from recordings in conscious rats showed that DA cells retain responses to predicted reward after responses to conditioned cues have developed, at least early in training. This contrasts with previous TD models that predict a gradual stepwise shift in latency with responses to rewards lost before responses develop to the conditioned cue. By exploring the TD parameter space, we demonstrate that the persistent reward responses of DA cells during conditioning are only accurately replicated by a TD model with long-lasting eligibility traces (nonzero values for the parameter lambda) and low learning rate (alpha). These physiological constraints for TD parameters suggest that eligibility traces and low per-trial rates of plastic modification may be essential features of neural circuits for reward learning in the brain. Such properties enable rapid but stable initiation of learning when the number of stimulus-reward pairings is limited, conferring significant adaptive advantages in real-world environments.
Figures
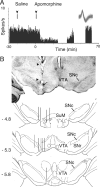
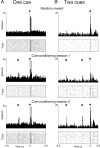
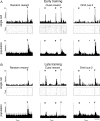



References
-
- Aebischer P, Schultz W (1984) The activity of pars compacta neurons of the monkey substantia nigra is depressed by apomorphine. Neurosci Lett 50: 25-29. - PubMed
-
- Aghajanian GK, Bunney BS (1977) Dopamine “autoreceptors”: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol 297: 1-7. - PubMed
-
- Barto AG (1995) Adaptive critics and the basal ganglia. In: Models of information processing in the basal ganglia (Houk JC, Davis JL, Beiser DG, eds), pp 215-232. Cambridge, MA: MIT.
-
- Barto AG, Sutton RS (1982) Simulation of anticipatory responses in classical-conditioning by a neuron-like adaptive element. Behav Brain Res 4: 221-235. - PubMed
-
- Bunney BS, Aghajanian GK, Roth RH (1973) Comparison of effects of l-dopa, amphetamine and apomorphine on firing rate of rat dopaminergic neurones. Nat New Biol 245: 123-125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
