Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse
- PMID: 15988013
- PMCID: PMC1168806
- DOI: 10.1128/MCB.25.14.5973-5984.2005
Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse
Abstract
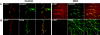
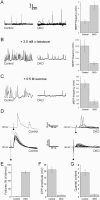
In cultured hippocampal neurons, synaptogenesis is largely independent of synaptic transmission, while several accounts in the literature indicate that synaptogenesis at cholinergic neuromuscular junctions in mammals appears to partially depend on synaptic activity. To systematically examine the role of synaptic activity in synaptogenesis at the neuromuscular junction, we investigated neuromuscular synaptogenesis and neurotransmitter release of mice lacking all synaptic vesicle priming proteins of the Munc13 family. Munc13-deficient mice are completely paralyzed at birth and die immediately, but form specialized neuromuscular endplates that display typical synaptic features. However, the distribution, number, size, and shape of these synapses, as well as the number of motor neurons they originate from and the maturation state of muscle cells, are profoundly altered. Surprisingly, Munc13-deficient synapses exhibit significantly increased spontaneous quantal acetylcholine release, although fewer fusion-competent synaptic vesicles are present and nerve stimulation-evoked secretion is hardly elicitable and strongly reduced in magnitude. We conclude that the residual transmitter release in Munc13-deficient mice is not sufficient to sustain normal synaptogenesis at the neuromuscular junction, essentially causing morphological aberrations that are also seen upon total blockade of neuromuscular transmission in other genetic models. Our data confirm the importance of Munc13 proteins in synaptic vesicle priming at the neuromuscular junction but indicate also that priming at this synapse may differ from priming at glutamatergic and gamma-aminobutyric acid-ergic synapses and is partly Munc13 independent. Thus, non-Munc13 priming proteins exist at this synapse or vesicle priming occurs in part spontaneously: i.e., without dedicated priming proteins in the release machinery.
Figures





References
-
- Allan, D. W., and J. J. Greer. 1997. Development of phrenic motoneuron morphology in the fetal rat. J. Comp. Neurol. 382:469-479. - PubMed
-
- Aravamudan, B., T. Fergestad, W. S. Davis, C. K. Rodesch, and K. Broadie. 1999. Drosophila UNC-13 is essential for synaptic transmission. Nat. Neurosci. 2:965-971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
