Activation of a nuclear Cdc2-related kinase within a mitogen-activated protein kinase-like TDY motif by autophosphorylation and cyclin-dependent protein kinase-activating kinase
- PMID: 15988018
- PMCID: PMC1168834
- DOI: 10.1128/MCB.25.14.6047-6064.2005
Activation of a nuclear Cdc2-related kinase within a mitogen-activated protein kinase-like TDY motif by autophosphorylation and cyclin-dependent protein kinase-activating kinase
Abstract
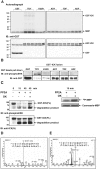
Male germ cell-associated kinase (MAK) and intestinal cell kinase (ICK) are nuclear Cdc2-related kinases with nearly identical N-terminal catalytic domains and more divergent C-terminal noncatalytic domains. The catalytic domain is also related to mitogen-activated protein kinases (MAPKs) and contains a corresponding TDY motif. Nuclear localization of ICK requires subdomain XI and interactions of the conserved Arg-272, but not kinase activity or, surprisingly, any of the noncatalytic domain. Further, nuclear localization of ICK is required for its activation. ICK is activated by dual phosphorylation of the TDY motif. Phosphorylation of Tyr-159 in the TDY motif requires ICK autokinase activity but confers only basal kinase activity. Full activation requires additional phosphorylation of Thr-157 in the TDY motif. Coexpression of ICK with constitutively active MEK1 or MEK5 fails to increase ICK phosphorylation or activity, suggesting that MEKs are not involved. ICK and MAK are related to Ime2p in budding yeast, and cyclin-dependent protein kinase-activating kinase Cak1p has been placed genetically upstream of Ime2p. Recombinant Cak1p phosphorylates Thr-157 in the TDY motif of recombinant ICK and activates its activity in vitro. Coexpression of ICK with wild-type CAK1 but not kinase-inactive CAK1 in cells also increases ICK phosphorylation and activity. Our studies establish ICK as the prototype for a new group of MAPK-like kinases requiring dual phosphorylation at TDY motifs.
Figures









References
-
- Abe, M. K., K. T. Kahle, M. P. Saelzler, K. Orth, J. E. Dixon, and M. R. Rosner. 2001. ERK7 is an autoactivated member of the MAPK family. J. Biol. Chem. 276:21272-21279. - PubMed
-
- Abe, S., T. Yagi, S. Ishiyama, M. Hiroe, F. Marumo, and Y. Ikawa. 1995. Molecular cloning of a novel serine/threonine kinase, MRK, possibly involved in cardiac development. Oncogene 11:2187-2195. - PubMed
-
- Alvarez, M., X. Estivill, and S. de la Luna. 2003. DYRK1A accumulates in splicing speckles through a novel targeting signal and induces speckle disassembly. J. Cell Sci. 116:3099-3107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
