Structural dynamics of alpha-actinin-vinculin interactions
- PMID: 15988023
- PMCID: PMC1168820
- DOI: 10.1128/MCB.25.14.6112-6122.2005
Structural dynamics of alpha-actinin-vinculin interactions
Erratum in
- Mol Cell Biol. 2007 Aug;27(15):5606
Abstract
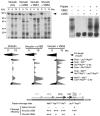
Alpha-actinin and vinculin orchestrate reorganization of the actin cytoskeleton following the formation of adhesion junctions. alpha-Actinin interacts with vinculin through the binding of an alpha-helix (alphaVBS) present within the R4 spectrin repeat of its central rod domain to vinculin's N-terminal seven-helical bundle domain (Vh1). The Vh1:alphaVBS structure suggests that alphaVBS first unravels from its buried location in the triple-helical R4 repeat to allow it to bind to vinculin. alphaVBS binding then induces novel conformational changes in the N-terminal helical bundle of Vh1, which disrupt its intramolecular association with vinculin's tail domain and which differ from the alterations in Vh1 provoked by the binding of talin. Surprisingly, alphaVBS binds to Vh1 in an inverted orientation compared to the binding of talin's VBSs to vinculin. Importantly, the binding of alphaVBS and talin's VBSs to vinculin's Vh1 domain appear to also trigger distinct conformational changes in full-length vinculin, opening up distant regions that are buried in the inactive molecule. The data suggest a model where vinculin's Vh1 domain acts as a molecular switch that undergoes distinct structural changes provoked by talin and alpha-actinin binding in focal adhesions versus adherens junctions, respectively.
Figures



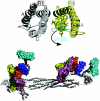
Comment in
-
Findings of misconduct in science/research misconduct.NIH Guide Grants Contracts (Bethesda). 2011 Jun 17:NOT-OD-11-084. NIH Guide Grants Contracts (Bethesda). 2011. PMID: 21714172 Free PMC article. No abstract available.
-
Findings of Misconduct in Science/Research Misconduct.Fed Regist. 2011 Jun 9;76(111):33763-33764. Fed Regist. 2011. PMID: 27737203 Free PMC article. No abstract available.
References
-
- Altmann, S. M., R. G. Grunberg, P. F. Lenne, J. Ylanne, A. Raae, K. Herbert, M. Saraste, M. Nilges, and J. K. Horber. 2002. Pathways and intermediates in forced unfolding of spectrin repeats. Structure 10:1085-1096. - PubMed
-
- Bakolitsa, C., D. M. Cohen, L. A. Bankston, A. A. Bobkov, G. W. Cadwell, L. Jennings, D. R. Critchley, S. W. Craig, and R. C. Liddington. 2004. Structural basis for vinculin activation at sites of cell adhesion. Nature 430:583-586. - PubMed
-
- Bakolitsa, C., J. M. de Pereda, C. R. Bagshaw, D. R. Critchley, and R. C. Liddington. 1999. Crystal structure of the vinculin tail suggests a pathway for activation. Cell 99:603-613. - PubMed
-
- Borgon, R. A., C. Vonrhein, G. Bricogne, P. R. J. Bois, and T. Izard. 2004. Crystal structure of human vinculin. Structure 12:1189-1197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
