Metabolon disruption: a mechanism that regulates bicarbonate transport
- PMID: 15990874
- PMCID: PMC1176462
- DOI: 10.1038/sj.emboj.7600736
Metabolon disruption: a mechanism that regulates bicarbonate transport
Abstract
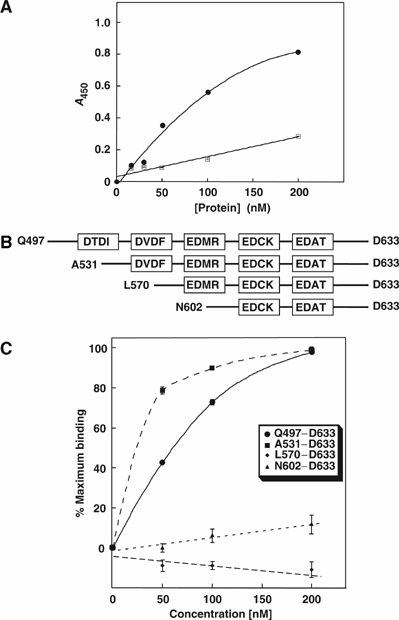
Carbonic anhydrases (CA) catalyze the reversible conversion of CO2 to HCO3-. Some bicarbonate transporters bind CA, forming a complex called a transport metabolon, to maximize the coupled catalytic/transport flux. SLC26A6, a plasma membrane Cl-/HCO3- exchanger with a suggested role in pancreatic HCO3- secretion, was found to bind the cytoplasmic enzyme CAII. Mutation of the identified CAII binding (CAB) site greatly reduced SLC26A6 activity, demonstrating the importance of the interaction. Regulation of SLC26A6 bicarbonate transport by protein kinase C (PKC) was investigated. Angiotensin II (AngII), which activates PKC, decreased Cl-/HCO3- exchange in cells coexpressing SLC26A6 and AT1a-AngII receptor. Activation of PKC reduced SLC26A6/CAII association in immunoprecipitates. Similarly, PKC activation displaced CAII from the plasma membrane, as monitored by immunofluorescence. Finally, mutation of a PKC site adjacent to the SLC26A6 CAB site rendered the transporter unresponsive to PKC. PKC therefore reduces CAII/SLC26A6 interaction, reducing bicarbonate transport rate. Taken together, our data support a mechanism for acute regulation of membrane transport: metabolon disruption.
Figures


References
-
- Alvarez BV, Fujinaga J, Casey JR (2001a) Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circ Res 89: 1246–1253 - PubMed
-
- Alvarez BV, Loiselle FB, Supuran CT, Schwartz GJ, Casey JR (2003) Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry 42: 12321–12329 - PubMed
-
- Alvarez L, Fanjul M, Carter N, Hollande E (2001b) Carbonic anhydrase II associated with plasma membrane in a human pancreatic duct cell line (CAPAN-1). J Histochem Cytochem 49: 1045–1053 - PubMed
-
- Aravind L, Koonin EV (2000) The STAS domain—a link between anion transporters and antisigma-factor antagonists. Curr Biol 10: R53–R55 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

