A short guided tour through functional and structural features of saposin-like proteins
- PMID: 15992358
- PMCID: PMC1175101
- DOI: 10.1042/BJ20050051
A short guided tour through functional and structural features of saposin-like proteins
Abstract
SAPLIPs (saposin-like proteins) are a diverse family of lipid-interacting proteins that have various and only partly understood, but nevertheless essential, cellular functions. Their existence is conserved in phylogenetically most distant organisms, such as primitive protozoa and mammals. Owing to their remarkable sequence variability, a common mechanism for their actions is not known. Some shared principles beyond their diversity have become evident by analysis of known three-dimensional structures. Whereas lipid interaction is the basis for their functions, the special cellular tasks are often defined by interaction partners other than lipids. Based on recent findings, this review summarizes phylogenetic relations, function and structural features of the members of this family.
Figures


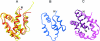
References
-
- Munford R. S., Sheppard P. O., O'Hara P. J. Saposin-like proteins (SAPLIP) carry out diverse functions on a common backbone structure. J. Lipid Res. 1995;36:1653–1663. - PubMed
-
- Munford R. S., Hunter J. P. Acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides, has phospholipase, lysophospholipase, diacylglycerollipase, and acyltransferase activities in vitro. J. Biol. Chem. 1992;267:10116–10121. - PubMed
-
- Kishimoto Y., Hiraiwa M., O'Brien J. S. Saposins: structure, function, distribution, and molecular genetics. J. Lipid Res. 1992;33:1255–1267. - PubMed
-
- Schuette C. G., Pierstorff B., Huettler S., Sandhoff K. Sphingolipid activator proteins: proteins with complex functions in lipid degradation and skin biogenesis. Glycobiology. 2001;11:81R–90R. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

