The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels
- PMID: 15994187
- PMCID: PMC1474200
- DOI: 10.1113/jphysiol.2005.085951
The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels
Abstract
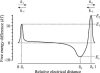
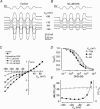
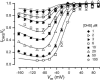
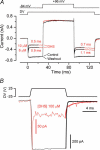
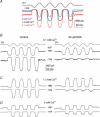
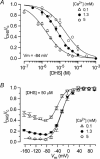
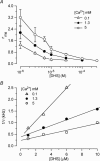
The most serious side-effect of the widely used aminoglycoside antibiotics is irreversible intracellular damage to the auditory and vestibular hair cells of the inner ear. The mechanism of entry into the hair cells has not been unequivocally resolved. Here we report that extracellular dihydrostreptomycin not only blocks the mechano-electrical transducer channels of mouse outer hair cells at negative membrane potentials, as previously shown, but also enters the cells through these channels, which are located in the cells' mechanosensory hair bundles. The voltage-dependent blocking kinetics indicate an open-channel block mechanism, which can be well described by a two barrier-one binding site model, quantifying the antibiotic's block of the channel as well as its permeation in terms of the associated rate constants. The results identify the open transducer channels as the main route for aminoglycoside entry. Intracellularly applied dihydrostreptomycin also blocks the transducer channels, but at positive membrane potentials. However, the potency of the block was two orders of magnitude lower than that due to extracellular dihydrostreptomycin. Extracellular Ca2+ increases the free energy of the barrier nearest the extracellular side and of the binding site for dihydrostreptomycin. This reduces both the entry of dihydrostreptomycin into the channel and the channel's affinity for the drug. In vivo, where the extracellular Ca2+ concentration in the endolymph surrounding the hair bundles is < 100 microM, we predict that some 9000 dihydrostreptomycin molecules per second enter each hair cell at therapeutic drug concentrations.
Figures


Comment in
-
Aminoglycoside ototoxicity: permeant drugs cause permanent hair cell loss.J Physiol. 2005 Sep 1;567(Pt 2):359-60. doi: 10.1113/jphysiol.2005.094474. Epub 2005 Jul 21. J Physiol. 2005. PMID: 16037084 Free PMC article. No abstract available.
References
-
- Bernard C. Action of streptomycin and calcium on the apical membrane of hair cells of the frog isolated semicircular canal. Acta Otolaryngol. 1983;96:21–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

