Negative regulation of CXCR4-mediated chemotaxis by the lipid phosphatase activity of tumor suppressor PTEN
- PMID: 15994292
- PMCID: PMC1895312
- DOI: 10.1182/blood-2004-08-3362
Negative regulation of CXCR4-mediated chemotaxis by the lipid phosphatase activity of tumor suppressor PTEN
Abstract
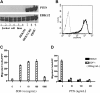
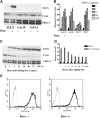
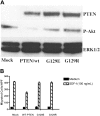
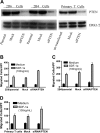
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a multifunctional tumor suppressor, has been shown to play a regulatory role in cell migration. Dictyostelium discoideum cells lacking PTEN exhibited impaired migration toward chemoattractant gradients. In the present study, we investigated the involvement of PTEN in chemotaxis of mammalian cells by examining PTEN-null Jurkat T cells. We observed that, in contrast to observations made in D discoideum, PTEN-null Jurkat T cells exhibited potent chemotactic responses to the chemokine stromal cell-derived factor 1alpha (SDF-1alpha), indicating that PTEN was not requisite for CXC chemokine receptor 4 (CXCR4)-mediated chemotaxis of Jurkat cells. Conversely, reconstitution of PTEN in Jurkat cells by using a tetracycline (Tet-on)-inducible expression system down-regulated CXCR4-mediated chemotaxis. Furthermore, we established the lipid phosphatase activity of PTEN as essential for its inhibitory effect on chemotaxis. In addition, using PTEN-expressing T-cell lines and primary T cells, we demonstrated that down-regulation of PTEN expression with vector-based small interfering RNAs (siRNAs) enhanced CXCR4-mediated chemotaxis. Based on these results, we conclude that PTEN expression negatively regulates chemotaxis of lymphoid mammalian cells via its lipid phosphatase activity. Our findings may account for the reported increase in metastatic activity of PTEN-null tumor cells.
Figures


Similar articles
-
Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha.J Biol Chem. 2005 Jun 10;280(23):22473-81. doi: 10.1074/jbc.M500963200. Epub 2005 Mar 31. J Biol Chem. 2005. PMID: 15802268
-
SHP2 and cbl participate in alpha-chemokine receptor CXCR4-mediated signaling pathways.Blood. 2001 Feb 1;97(3):608-15. doi: 10.1182/blood.v97.3.608. Blood. 2001. PMID: 11157475
-
Differential regulation of CXCR4-mediated T-cell chemotaxis and mitogen-activated protein kinase activation by the membrane tyrosine phosphatase, CD45.J Biol Chem. 2003 Mar 14;278(11):9536-43. doi: 10.1074/jbc.M211803200. Epub 2003 Jan 8. J Biol Chem. 2003. PMID: 12519755
-
Development and characterisation of tetracycline-regulated phosphoinositide 3-kinase mutants: assessing the role of multiple phosphoinositide 3-kinases in chemokine signaling.J Immunol Methods. 2003 Feb;273(1-2):29-41. doi: 10.1016/s0022-1759(02)00416-7. J Immunol Methods. 2003. PMID: 12535795 Review.
-
PI 3-kinases and PTEN: how opposites chemoattract.Cell. 2002 May 31;109(5):541-4. doi: 10.1016/s0092-8674(02)00765-1. Cell. 2002. PMID: 12062096 Review.
Cited by
-
Calcium Phosphate Coating Prepared by Microarc Oxidation Affects hTERT Expression, Molecular Presentation, and Cytokine Secretion in Tumor-Derived Jurkat T Cells.Materials (Basel). 2020 Sep 27;13(19):4307. doi: 10.3390/ma13194307. Materials (Basel). 2020. PMID: 32992463 Free PMC article.
-
Video-rate two-photon imaging of mouse footpad - a promising model for studying leukocyte recruitment dynamics during inflammation.Inflamm Res. 2008 Mar;57(3):93-6. doi: 10.1007/s00011-007-7195-y. Inflamm Res. 2008. PMID: 18213448 Free PMC article.
-
Loss of PTEN permits CXCR4-mediated tumorigenesis through ERK1/2 in prostate cancer cells.Mol Cancer Res. 2011 Jan;9(1):90-102. doi: 10.1158/1541-7786.MCR-10-0235. Epub 2010 Nov 12. Mol Cancer Res. 2011. PMID: 21076047 Free PMC article.
-
A beta version of life: p110β takes center stage.Oncotarget. 2010 Dec;1(8):729-733. doi: 10.18632/oncotarget.207. Oncotarget. 2010. PMID: 21321382 Free PMC article. Review.
-
PtdIns(3,4,5)P(3)-dependent and -independent roles for PTEN in the control of cell migration.Curr Biol. 2007 Jan 23;17(2):115-25. doi: 10.1016/j.cub.2006.12.026. Curr Biol. 2007. PMID: 17240336 Free PMC article.
References
-
- Locati M, Murphy PM. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu Rev Med. 1999;50: 425-440. - PubMed
-
- Meili R, Firtel RA. Two poles and a compass. Cell. 2003;114: 153-156. - PubMed
-
- Laudanna C, Kim JY, Constantin G, Butcher E. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002;186: 37-46. - PubMed
-
- Mellado M, Rodriguez-Frade JM, Manes S, Martinez AC. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19: 397-421. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

