A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates
- PMID: 15994776
- PMCID: PMC1168733
- DOI: 10.1128/JVI.79.14.8828-8834.2005
A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates
Abstract
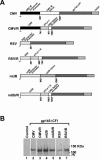
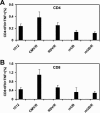
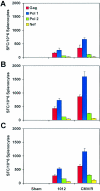
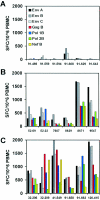
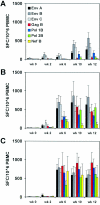
Plasmid DNA vaccines elicit potent and protective immune responses in numerous small-animal models of infectious diseases. However, their immunogenicity in primates appears less potent. Here we investigate a novel approach that optimizes regulatory elements in the plasmid backbone to improve the immunogenicity of DNA vaccines. Among various regions analyzed, we found that the addition of a regulatory sequence from the R region of the long terminal repeat from human T-cell leukemia virus type 1 (HTLV-1) to the cytomegalovirus (CMV) enhancer/promoter increased transgene expression 5- to 10-fold and improved cellular immune responses to human immunodeficiency virus type 1 (HIV-1) antigens. In cynomolgus monkeys, DNA vaccines containing the CMV enhancer/promoter with the HTLV-1 R region (CMV/R) induced markedly higher cellular immune responses to HIV-1 Env from clades A, B, and C and to HIV-1 Gag-Pol-Nef compared with the parental DNA vaccines. These data demonstrate that optimization of specific regulatory elements can substantially improve the immunogenicity of DNA vaccines encoding multiple antigens in small animals and in nonhuman primates. This strategy could therefore be explored as a potential method to enhance DNA vaccine immunogenicity in humans.
Figures
References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S.P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-K. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of clinical AIDS in rhesus monkeys by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Attal, J., M. C. Theron, F. Taboit, M. Cajero-Juarez, G. Kann, P. Bolifraud, and L. M. Houdebine. 1996. The RU5 (′R') region from human leukaemia viruses (HTLV-1) contains an internal ribosome entry site (IRES)-like sequence. FEBS Lett. 392:220-224. - PubMed
-
- Barouch, D. H., S. Santra, T. D. Steenbeke, X. X. Zheng, H. C. Perry, M.-E. Davies, D. C. Freed, A. Craiu, T. B. Strom, J. W. Shiver, and N. L. Letvin. 1998. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J. Immunol. 161:1875-1882. - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M.-E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
-
- Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168:562-568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

