The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates
- PMID: 15994792
- PMCID: PMC1168791
- DOI: 10.1128/JVI.79.14.8979-8990.2005
The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates
Abstract
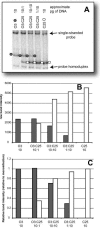
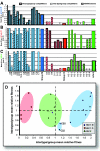
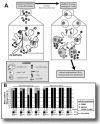
The main (M) group of human immunodeficiency virus type 1 (HIV-1) is responsible for the global AIDS epidemic while HIV-1 group O (outlier) and HIV type 2 are endemic only in west and central Africa. The failure of HIV-2 and especially HIV-1 group O to spread following the initial zoonotic jumps is not well understood. This study was designed to examine the relative replicative capacities between these human lentiviruses. A pairwise competition experiment was performed with peripheral blood mononuclear cells with eight HIV-2 isolates, 6 group O viruses, and 15 group M viruses of subtype A (2 viruses), B (5 viruses), C (4 viruses), D (2 viruses) and CRF01_AE (2 viruses). HIV-1 group M isolates of any subtype were typically 100-fold-more fit than group O or HIV-2 strains when competed in peripheral blood mononuclear cells from various humans. This order in replicative fitness was also observed when virus pairs were added to human dendritic cells and then cocultured with primary, quiescent T cells, which is the model for HIV-1 transmission. These results suggest that reduced replicative and transmission fitness may be contributing to the low prevalence and limited geographical spread of HIV-2 and group O HIV-1 in the human population.
Figures



References
-
- Anderson, R. M., and R. M. May. 1996. The population biology of the interaction between HIV-1 and HIV-2: coexistence or competitive exclusion? AIDS 10:1663-1673. - PubMed
-
- Anderson, R. M., and R. M. May. 1991. Infectious diseases of humans. Oxford University Press, Oxford, United Kingdom.
-
- Arts, E. J., and M. E. Quinones-Mateu. 2003. Sorting out the complexities of HIV-1 fitness. AIDS 17:780-781. - PubMed
-
- Ball, S. C., A. Abraha, K. R. Collins, A. J. Marozsan, H. Baird, M. E. Quiñones-Mateu, A. Penn-Nicholson, M. Murray, N. Richard, M. Lobritz, P. A. Zimmerman, T. Kawamura, A. Blauvelt, and E. J. Arts. 2003. Comparing the ex vivo fitness of CCR5-tropic human immunodeficiency virus type 1 isolates of subtypes B and C. J. Virol. 77:1021-1038. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

