Analysis of human immunodeficiency virus type 1 Gag ubiquitination
- PMID: 15994808
- PMCID: PMC1168789
- DOI: 10.1128/JVI.79.14.9134-9144.2005
Analysis of human immunodeficiency virus type 1 Gag ubiquitination
Abstract
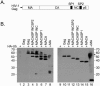
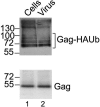
Ubiquitin is important for the release of human immunodeficiency virus type 1 (HIV-1) and several other retroviruses, but the functional significance of Gag ubiquitination is unknown. To address this problem, we decided to analyze Gag ubiquitination in detail. A low percentage of the HIV-1 p6 protein has previously been shown to be ubiquitinated, and published mutagenesis data suggested that Gag ubiquitination is largely lost upon mutation of the two lysine residues in p6. In this study, we show that Gag proteins lacking the p6 domain or the two lysine residues within p6 are ubiquitinated at levels comparable to those of the wild-type Gag protein. We detected monoubiquitinated forms of the matrix (MA), capsid (CA), and nucleocapsid (NC) proteins in mature virus preparations. Protease digestion of Gag polyproteins extracted from immature virions indicated that ubiquitinated MA, CA, and possibly NC are as abundant as ubiquitinated p6. The HIV-1 late-domain motifs PTAP and LRSLF were not required for Gag ubiquitination, and mutation of the PTAP motif even resulted in an increase in the amount of Gag-Ub conjugates detected. Finally, at steady state, ubiquitinated Gag proteins were not enriched in either membrane-associated or virus-derived Gag fractions. In summary, these results indicate that HIV-1 Gag can be monoubiquitinated in all domains and that ubiquitination of lysine residues outside p6 may thus contribute to viral release and/or infectivity.
Figures





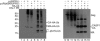

Similar articles
-
Cumulative mutations of ubiquitin acceptor sites in human immunodeficiency virus type 1 gag cause a late budding defect.J Virol. 2006 Jul;80(13):6267-75. doi: 10.1128/JVI.02177-05. J Virol. 2006. PMID: 16775314 Free PMC article.
-
Ubiquitination of HIV-1 and MuLV Gag.Virology. 2000 Dec 5;278(1):111-21. doi: 10.1006/viro.2000.0648. Virology. 2000. PMID: 11112487
-
Ubiquitination of human immunodeficiency virus type 1 Gag is highly dependent on Gag membrane association.J Virol. 2007 Sep;81(17):9193-201. doi: 10.1128/JVI.00044-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609272 Free PMC article.
-
The PTAP sequence within the p6 domain of human immunodeficiency virus type 1 Gag regulates its ubiquitination and MHC class I antigen presentation.J Immunol. 2011 May 15;186(10):5706-18. doi: 10.4049/jimmunol.1003764. Epub 2011 Apr 11. J Immunol. 2011. PMID: 21482733
-
The role of viral and cellular proteins in the budding of human immunodeficiency virus.Acta Virol. 2006;50(2):75-85. Acta Virol. 2006. PMID: 16808324 Review.
Cited by
-
Cumulative mutations of ubiquitin acceptor sites in human immunodeficiency virus type 1 gag cause a late budding defect.J Virol. 2006 Jul;80(13):6267-75. doi: 10.1128/JVI.02177-05. J Virol. 2006. PMID: 16775314 Free PMC article.
-
Ubiquitination and SUMOylation in HIV Infection: Friends and Foes.Curr Issues Mol Biol. 2020;35:159-194. doi: 10.21775/cimb.035.159. Epub 2019 Aug 18. Curr Issues Mol Biol. 2020. PMID: 31422939 Free PMC article. Review.
-
Tsg101 chaperone function revealed by HIV-1 assembly inhibitors.Nat Commun. 2017 Nov 9;8(1):1391. doi: 10.1038/s41467-017-01426-2. Nat Commun. 2017. PMID: 29123089 Free PMC article.
-
Functional hierarchy of two L domains in porcine endogenous retrovirus (PERV) that influence release and infectivity.Virology. 2008 Jun 5;375(2):637-45. doi: 10.1016/j.virol.2008.02.017. Epub 2008 Mar 19. Virology. 2008. PMID: 18355887 Free PMC article.
-
The matrix protein of human immunodeficiency virus is cleaved and packed into virion cores.Dokl Biol Sci. 2006 May-Jun;408:249-52. doi: 10.1134/s0012496606030136. Dokl Biol Sci. 2006. PMID: 16909991 No abstract available.
References
-
- Abacioglu, Y. H., T. R. Fouts, J. D. Laman, E. Claassen, S. H. Pincus, J. P. Moore, C. A. Roby, R. Kamin-Lewis, and G. K. Lewis. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res. Hum. Retrovir. 10:371-381. - PubMed
-
- Bohne, J., and H. G. Krausslich. 2004. Mutation of the major 5′ splice site renders a CMV-driven HIV-1 proviral clone Tat-dependent: connections between transcription and splicing. FEBS Lett. 563:113-118. - PubMed
-
- Bouamr, F., J. A. Melillo, M. Q. Wang, K. Nagashima, M. de Los Santos, A. Rein, and S. P. Goff. 2003. PPPYVEPTAP motif is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101. J. Virol. 77:11882-11895. - PMC - PubMed
-
- Freed, E. O. 1998. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 251:1-15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

