Genetic variability and molecular evolution of the human respiratory syncytial virus subgroup B attachment G protein
- PMID: 15994810
- PMCID: PMC1168771
- DOI: 10.1128/JVI.79.14.9157-9167.2005
Genetic variability and molecular evolution of the human respiratory syncytial virus subgroup B attachment G protein
Abstract
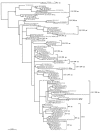
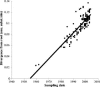
Human respiratory syncytial virus (HRSV) is the most important cause of acute respiratory disease in infants. Two major subgroups (A and B) have been identified based on antigenic differences in the attachment G protein. Antigenic variation between and within the subgroups may contribute to reinfections with these viruses by evading the host immune responses. To investigate the circulation patterns and mechanisms by which HRSV-B viruses evolve, we analyzed the G protein genetic variability of subgroup B sequences isolated over a 45-year period, including 196 Belgian strains obtained over 22 epidemic seasons (1982 to 2004). Our study revealed that the HRSV-B evolutionary rate (1.95 x 10(-3) nucleotide substitutions/site/year) is similar to that previously estimated for HRSV-A (1.83 x 10(-3) nucleotide substitutions/site/year). However, natural HRSV-B isolates appear to accommodate more drastic changes in their attachment G proteins. The most recent common ancestor of the currently circulating subgroup B strains was estimated to date back to around the year 1949. The divergence between the two major subgroups was calculated to have occurred approximately 350 years ago. Furthermore, we have identified 12 positively selected sites in the G protein ectodomain, suggesting that immune-driven selective pressure operates in certain codon positions. HRSV-A and -B strains have similar phylodynamic patterns: both subgroups are characterized by global spatiotemporal strain dynamics, where the high infectiousness of HRSV permits the rapid geographic spread of novel strain variants.
Figures


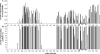

References
-
- Anderson, L. J., J. C. Hierholzer, C. Tsou, R. M. Hendry, B. F. Fernie, Y. Stone, and K. McIntosh. 1985. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 151:626-633. - PubMed
-
- Cane, P. A., D. A. Matthews, and C. R. Pringle. 1991. Identification of variable domains of the attachment (G) protein of subgroup A respiratory syncytial viruses. J. Gen. Virol. 72:2091-2096. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

