Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus
- PMID: 15994816
- PMCID: PMC1168785
- DOI: 10.1128/JVI.79.14.9217-9227.2005
Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus
Erratum in
- J Virol. 2005 Sep;79(17)11552. Wolinksy, Steven [corrected to Wolinsky, Steven]
Abstract
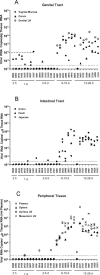
In the current global AIDS pandemic, more than half of new human immunodeficiency virus type 1 (HIV-1) infections are acquired by women through intravaginal HIV exposure. For this study, we explored pathogenesis issues relevant to the development of effective vaccines to prevent infection by this route, using an animal model in which female rhesus macaques were exposed intravaginally to a high dose of simian immunodeficiency virus (SIV). We examined in detail the events that transpire from hours to a few days after intravaginal SIV exposure through week 4 to provide a framework for understanding the propagation, dissemination, and establishment of infection in lymphatic tissues (LTs) during the acute stage of infection. We show that the mucosal barrier greatly limits the infection of cervicovaginal tissues, and thus the initial founder populations of infected cells are small. While there was evidence of rapid dissemination to distal sites, we also show that continuous seeding from an expanding source of production at the portal of entry is likely critical for the later establishment of a productive infection throughout the systemic LTs. The initially small founder populations and dependence on continuous seeding to establish a productive infection in systemic LTs define a small window of maximum vulnerability for the virus in which there is an opportunity for the host, vaccines, or other interventions to prevent or control infection.
Figures






References
-
- Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099-3118. - PMC - PubMed
-
- Dailey, P. J., M. Zamround, R. Kelso, J. Kolberg, and M. Urdea. 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected macaques using a branched DNA (bDNA) signal amplification assay. Presented at the 13th Annual Symposium on Nonhuman Primate Models of AIDS, Monterey, Calif.
-
- Fokin, A. A., F. Robicsek, and T. N. Masters. 2000. Transport of viral-size particulate matter after intravenous versus intralymphatic entry. Microcirculation 7:357-365. - PubMed
-
- Hendrickx, A. G., and M. A. Cukierski. 1987. Reproductive and developmental toxicology in nonhuman primates, p. 73-88. In C. R. Graham (ed.), Preclinical safety of biotechnology products intended for human use. Alan R. Liss, Inc., New York, N.Y. - PubMed
-
- Hirsch, V., N. Riedel, and J. I. Mullins. 1987. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell 49:307-319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

