Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors
- PMID: 15995945
- PMCID: PMC1224529
- DOI: 10.1086/432648
Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors
Abstract
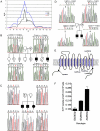
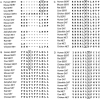
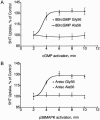
Autism is a spectrum of neurodevelopmental disorders with a primarily genetic etiology exhibiting deficits in (1) development of language and (2) social relationships and (3) patterns of repetitive, restricted behaviors or interests and resistance to change. Elevated platelet serotonin (5-HT) in 20%-25% of cases and efficacy of selective 5-HT reuptake inhibitors (SSRIs) in treating anxiety, depression, and repetitive behaviors points to the 5-HT transporter (5-HTT; SERT) as a strong candidate gene. Association studies involving the functional insertion/deletion polymorphism in the promoter (5-HTTLPR) and a polymorphism in intron 2 are inconclusive, possibly because of phenotypic heterogeneity. Nonetheless, mounting evidence for genetic linkage of autism to the chromosome 17q11.2 region that harbors the SERT locus (SLC6A4) supports a genetic effect at or near this gene. We confirm recent reports of sex-biased genetic effects in 17q by showing highly significant linkage driven by families with only affected males. Association with common alleles fails to explain observed linkage; therefore, we hypothesized that preferential transmission of multiple alleles does explain it. From 120 families, most contributing to linkage at 17q11.2, we found four coding substitutions at highly conserved positions and 15 other variants in 5' noncoding and other intronic regions transmitted in families exhibiting increased rigid-compulsive behaviors. In the aggregate, these variants show significant linkage to and association with autism. Our data provide strong support for a collection of multiple, often rare, alleles at SLC6A4 as imposing risk of autism.
Figures

References
Web Resources
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for SLC6A4 [accession number NM_001045])
-
- NIMH Center for Collaborative Genetic Studies on Mental Disorders, http://www.nimhgenetics.org/
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for autism, SLC6A4, ITGB3, Asperger syndrome, and OCD)
-
- Single Nucleotide Polymorphism, http://www.ncbi.nlm.nih.gov/SNP/ (for dbSNP numbers ss38318598, ss38318599, ss38318600, ss38318601, ss38318589, ss38318590, ss38318591, ss38318592, ss38318593, ss38318594, ss38318595, ss38318596, and ss38318597)
References
-
- Ashley-Koch A, Wolpert CM, Menold MM, Zaeem L, Basu S, Donnelly SL, Ravan SA, Powell CM, Qumsiyeh MB, Aylsworth AS, Vance JM, Gilbert JR, Wright HH, Abramson RK, DeLong GR, Cuccaro ML, Pericak-Vance MA (1999) Genetic studies of autistic disorder and chromosome 7. Genomics 61:227–236 - PubMed
-
- Auranen M, Nieminen T, Majuri S, Vanhala R, Peltonen L, Jarvela I (2000) Analysis of autism susceptibility gene loci on chromosomes 1p, 4p, 6q, 7q, 13q, 15q, 16p, 17q, 19q and 22q in Finnish multiplex families. Mol Psychiatry 5:320–322 - PubMed
-
- Bolton PF, Pickles A, Murphy M, Rutter M (1998) Autism, affective and other psychiatric disorders: patterns of familial aggregation. Psychol Med 28:385–395 - PubMed
-
- Buxbaum JD, Silverman J, Keddache M, Smith CJ, Hollander E, Ramoz N, Reichert JG (2004) Linkage analysis for autism in a subset families with obsessive-compulsive behaviors: evidence for an autism susceptibility gene on chromosome 1 and further support for susceptibility genes on chromosome 6 and 19. Mol Psychiatry 9:144–150 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

