Hypoxia aggravates lipopolysaccharide-induced lung injury
- PMID: 15996189
- PMCID: PMC1809432
- DOI: 10.1111/j.1365-2249.2005.02835.x
Hypoxia aggravates lipopolysaccharide-induced lung injury
Abstract
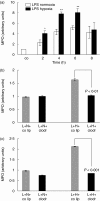
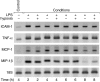
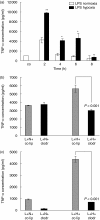
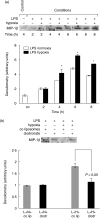
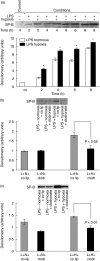
The animal model of inflammatory response induced by intratracheal application of lipopolysaccharide includes many typical features of acute lung injury or the acute respiratory distress syndrome. A number of experimental investigations have been performed to characterize the nature of this injury more effectively. In inflammatory conditions, hypoxia occurs frequently before and in parallel with pulmonary and non-pulmonary pathological events. This current study was designed to examine the in vivo effect of hypoxia as a potentially aggravating condition in endotoxin-induced lung injury. Lipopolysaccharide, 150 microg, was instilled intratracheally into rat lungs, and thereafter animals were exposed to either normoxia or hypoxia (10% oxygen). Lungs were collected 2, 4, 6 and 8 h later. Inflammatory response and tissue damage were evaluated by quantitative analysis of inflammatory cells and mediators, surfactant protein and vascular permeability. A significantly enhanced neutrophil recruitment was seen in lipopolysaccharide-animals exposed to hypoxia compared to lipopolysaccharide-animals under normoxia. This increased neutrophil accumulation was triggered by inflammatory mediators such as tumour necrosis factor-alpha and macrophage inflammatory protein-1beta, secreted by alveolar macrophages. Determination of vascular permeability and surfactant protein-B showed enhanced concentrations in lipopolysaccharide-lungs exposed to hypoxia, which was absent in animals previously alveolar macrophage-depleted. This study demonstrates that hypoxia aggravates lipopolysaccharide injury and therefore represents a second hit injury. The additional hypoxia-induced inflammatory reaction seems to be predominantly localized in the respiratory compartment, underlining the compartmentalized nature of the inflammatory response.
Figures


Comment in
-
One-hit, two-hit . . . is there really any benefit?Clin Exp Immunol. 2005 Aug;141(2):211-4. doi: 10.1111/j.1365-2249.2005.02853.x. Clin Exp Immunol. 2005. PMID: 15996184 Free PMC article. No abstract available.
Similar articles
-
Early tumor necrosis factor-alpha release from the pulmonary macrophage in lung ischemia-reperfusion injury.J Thorac Cardiovasc Surg. 2004 May;127(5):1502-8. doi: 10.1016/j.jtcvs.2003.08.019. J Thorac Cardiovasc Surg. 2004. PMID: 15116014
-
Acute Lung Injury in Response to Intratracheal Instillation of Lipopolysaccharide in an Animal Model of Emphysema Induced by Elastase.Inflammation. 2018 Feb;41(1):174-182. doi: 10.1007/s10753-017-0675-5. Inflammation. 2018. PMID: 28975419
-
Pulmonary aspiration: new therapeutic approaches in the experimental model.Anesthesiology. 2005 Sep;103(3):556-66. doi: 10.1097/00000542-200509000-00019. Anesthesiology. 2005. PMID: 16129981
-
Alveolar Hypoxia-Induced Pulmonary Inflammation: From Local Initiation to Secondary Promotion by Activated Systemic Inflammation.J Vasc Res. 2016;53(5-6):317-329. doi: 10.1159/000452800. Epub 2016 Dec 15. J Vasc Res. 2016. PMID: 27974708 Review.
-
Acute lung injury: how the lung inflammatory response works.Eur Respir J Suppl. 2003 Sep;44:22s-23s. doi: 10.1183/09031936.03.00000703a. Eur Respir J Suppl. 2003. PMID: 14582896 Review. No abstract available.
Cited by
-
Pulmonary contusion primes systemic innate immunity responses.J Trauma. 2009 Jul;67(1):14-21; discussion 21-2. doi: 10.1097/TA.0b013e31819ea600. J Trauma. 2009. PMID: 19590302 Free PMC article.
-
Acute hypoxia decreases E. coli LPS-induced cytokine production and NF-kappaB activation in alveolar macrophages.Respir Physiol Neurobiol. 2010 Jun 30;172(1-2):63-71. doi: 10.1016/j.resp.2010.05.006. Epub 2010 May 12. Respir Physiol Neurobiol. 2010. PMID: 20470909 Free PMC article.
-
Blunt Chest Trauma in Mice after Cigarette Smoke-Exposure: Effects of Mechanical Ventilation with 100% O2.PLoS One. 2015 Jul 30;10(7):e0132810. doi: 10.1371/journal.pone.0132810. eCollection 2015. PLoS One. 2015. PMID: 26225825 Free PMC article.
-
Alveolar hypoxia, alveolar macrophages, and systemic inflammation.Respir Res. 2009 Jun 22;10(1):54. doi: 10.1186/1465-9921-10-54. Respir Res. 2009. PMID: 19545431 Free PMC article. Review.
-
Hypoxia Exacerbates Inflammatory Signaling in Human Coronavirus OC43-Infected Lung Epithelial Cells.Biomolecules. 2025 Aug 8;15(8):1144. doi: 10.3390/biom15081144. Biomolecules. 2025. PMID: 40867589 Free PMC article.
References
-
- Ulich TR, Howard SC, Remick DG, et al. Intratracheal administration of endotoxin and cytokines. VIII. LPS induces E-selectin expression; anti-E-selectin and soluble E-selectin inhibit acute inflammation. Inflammation. 1994;18:389–98. - PubMed
-
- Ulich TR, Howard SC, Remick DG, et al. Intratracheal administration of endotoxin and cytokines. VI. Antiserum to CINC inhibits acute inflammation. Am J Physiol. 1995;268:L245–50. - PubMed
-
- Beck-Schimmer B, Schimmer RC, Warner RL, et al. Expression of lung vascular and airway ICAM-1 after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 1997;17:344–52. - PubMed
-
- van Helden HP, Kuijpers WC, Steenvoorden D, et al. Intratracheal aerosolization of endotoxin (LPS) in the rat: a comprehensive animal model to study adult (acute) respiratory distress syndrome. Exp Lung Res. 1997;23:297–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

