Regulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16+ monocytes in response to sepsis-related antigens
- PMID: 15996191
- PMCID: PMC1809439
- DOI: 10.1111/j.1365-2249.2005.02839.x
Regulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16+ monocytes in response to sepsis-related antigens
Abstract
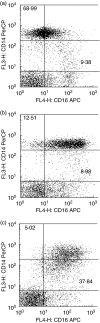
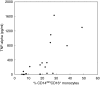
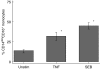
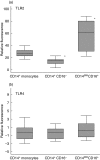
Rapid overproduction of proinflammatory cytokines are characteristic of sepsis. CD14(dim)CD16(+) monocytes are thought to be major producers of cytokine and have been shown to be elevated in septic patients. Toll-like receptors (TLR) are pattern recognition receptors important in mediating the innate immune response and their activation can lead to production of cytokines. Using whole blood culture and flow cytometry we have investigated TLR2 and TLR4 regulation after stimulation with sepsis-relevant antigens [lipopolysaccharide (LPS), Staphylococcal enterotoxin B (SEB) and peptidoglycan (PGN)]. The percentage of CD14(dim)CD16(+) monocyte population expanded at 20 h post-stimulation, after a rise in tumour necrosis factor (TNF)-alpha and interleukin (IL)-6 at 2 h. A strong positive correlation between the percentage of CD14(dim)CD16(+) monocytes and secreted TNF-alpha was demonstrated (r = 0.72). Furthermore, we were able to induce expansion of the CD14(dim)CD16(+) population to approximately 35% of all monocytes with the addition of recombinant TNF-alpha to the whole blood culture. TLR4 was found to be expressed 2.5 times higher on CD14(dim)CD16(+) compared to CD14(+) CD16(-) monocytes, while TLR2 expression was similar in both subpopulations. The CD14(dim)CD16(+) and CD14(+) CD16(-) monocyte populations were different in their response to various antigens. LPS down-regulated TLR4 by 4.9 times in CD16(+) monocytes compared to only 2.3 times in CD16(-) monocytes at 2 h. LPS was able to up-regulate TLR2 by 6.2 times after 2 h, with no difference between the subpopulations. LPS further up-regulated TLR2 by 18.4 times after 20 h only in the CD14(+) CD16(-) population. PGN and SEB induced no significant changes in TLR2 or TLR4 expression. We hypothesize that following exposure to bacterial antigens, subsequent TNF-alpha drives a differentiation of monocytes into a CD14(dim)CD16(+) subpopulation.
Figures



References
-
- Clermont G, Angus DC, Kalassian KG, et al. Reassessing the value of short-term mortality in sepsis: comparing conventional approaches to modeling. Crit Care Med. 2003;31:2627–33. - PubMed
-
- Schoenberg MH, Weiss M, Radermacher P. Outcome of patients with sepsis and septic shock after ICU treatment. Langenbecks Arch Surg. 1998;383:44–8. - PubMed
-
- Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. - PubMed
-
- Bannan J, Visvanathan K, Zabriskie JB. Structure and function of streptococcal and staphylococcal superantigens in septic shock. Infect Dis Clin North Am. 1999;13:387–96. ix. - PubMed
-
- Dalpke AH, Heeg K. Synergistic and antagonistic interactions between LPS and superantigens. J Endotoxin Res. 2003;9:51–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

