Induction of cytochrome P450 1A by cow milk-based formula: a comparative study between human milk and formula
- PMID: 15997229
- PMCID: PMC1576269
- DOI: 10.1038/sj.bjp.0706319
Induction of cytochrome P450 1A by cow milk-based formula: a comparative study between human milk and formula
Abstract
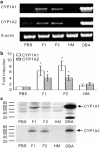
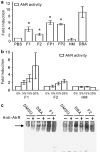
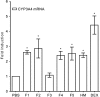
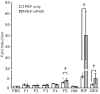
During the treatment of neonatal apnea, formula-fed infants, compared to breastfed infants, show nearly three-fold increase in clearance of caffeine, a substrate of cytochrome P450 1A (CYP1A) and in part CYP3A4. However, human milk is known to contain higher concentrations of environmental pollutants than infant formula, which are potent CYP1A inducers. To gain insight into the mechanism underlying this apparent contradiction, we characterized CYP1A and CYP3A4 induction by human milk and cow milk-based infant formula. The mRNA and protein expression of CYP1A1/1A2 were significantly induced by cow milk-based formula, but not by human milk, in HepG2 cells. Luciferase reporter assay demonstrated that cow milk-based formula but not human milk activated aryl hydrocarbon receptor (AhR) significantly. The cotreatment of 3,4-dimethoxyflavone, an AhR antagonist, abolished the formula-induced CYP1A expression. In addition, AhR activation by dibenzo[a,h]anthracene, a potent AhR agonist, was significantly suppressed by infant formula and even more by human milk. In contrast, CYP3A4 mRNA expression was only mildly induced by formula and human milk. Consistently, neither formula nor human milk substantially activated pregnane X receptor (PXR). Effects of whey and soy protein-based formulas on the AhR-CYP1A and the PXR-CYP3A4 pathways were similar to those of cow milk-based formula. In conclusion, infant formula, but not human milk, enhances in vitro CYP1A expression via an AhR-mediated pathway, providing a potential mechanistic basis for the increased caffeine elimination in formula-fed infants.
Figures


Similar articles
-
Environmental pollutants parathion, paraquat and bisphenol A show distinct effects towards nuclear receptors-mediated induction of xenobiotics-metabolizing cytochromes P450 in human hepatocytes.Toxicol Lett. 2015 Oct 1;238(1):43-53. doi: 10.1016/j.toxlet.2015.07.008. Epub 2015 Jul 18. Toxicol Lett. 2015. PMID: 26196221
-
Evaluation of human liver slices and reporter gene assays as systems for predicting the cytochrome p450 induction potential of drugs in vivo in humans.Pharm Res. 2006 Jan;23(1):56-69. doi: 10.1007/s11095-005-8812-5. Epub 2006 Nov 22. Pharm Res. 2006. PMID: 16328606
-
Role of CYP3A4 in the regulation of the aryl hydrocarbon receptor by omeprazole sulphide.Cell Signal. 2006 May;18(5):740-50. doi: 10.1016/j.cellsig.2005.07.007. Epub 2005 Aug 16. Cell Signal. 2006. PMID: 16109480
-
CYP1A in TCDD toxicity and in physiology-with particular reference to CYP dependent arachidonic acid metabolism and other endogenous substrates.Drug Metab Rev. 2006;38(1-2):291-335. doi: 10.1080/03602530600570107. Drug Metab Rev. 2006. PMID: 16684662 Review.
-
Infant formulas with increased concentrations of alpha-lactalbumin.Am J Clin Nutr. 2003 Jun;77(6):1555S-1558S. doi: 10.1093/ajcn/77.6.1555S. Am J Clin Nutr. 2003. PMID: 12812154 Review.
Cited by
-
Ontogeny of Drug-Metabolizing Enzymes.Methods Mol Biol. 2021;2342:551-593. doi: 10.1007/978-1-0716-1554-6_18. Methods Mol Biol. 2021. PMID: 34272706 Review.
-
Impact of Drug Treatment at Neonatal Ages on Variability of Drug Metabolism and Drug-drug Interactions in Adult Life.Curr Pharmacol Rep. 2017 Feb;3(1):1-9. doi: 10.1007/s40495-016-0078-6. Epub 2017 Jan 3. Curr Pharmacol Rep. 2017. PMID: 28344923 Free PMC article.
-
Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds.Curr Drug Metab. 2006 May;7(4):349-65. doi: 10.2174/138920006776873526. Curr Drug Metab. 2006. PMID: 16724925 Free PMC article. Review.
-
Functional evolution of the pregnane X receptor.Expert Opin Drug Metab Toxicol. 2006 Jun;2(3):381-97. doi: 10.1517/17425255.2.3.381. Expert Opin Drug Metab Toxicol. 2006. PMID: 16863441 Free PMC article. Review.
References
-
- ABRAHAM K., KNOLL A., ENDE M., PAPKE O., HELGE H. Intake, fecal excretion, and body burden of polychlorinated dibenzo-p-dioxins and dibenzofurans in breast-fed and formula-fed infants. Pediatr. Res. 1996;40:671–679. - PubMed
-
- AMERICAN ACADEMY OF PEDIATRICS WORK GROUP ON BREASTFEEDING Breastfeeding and the use of human milk. Pediatrics. 1997;100:1035–1039. - PubMed
-
- AMERICAN ACADEMY OF PEDIATRICS. COMMITTEE ON NUTRITION Soy protein-based formulas: recommendations for use in infant feeding. Pediatrics. 1998;101:148–153. - PubMed
-
- BARBANO D.M., CLARK J.L., DUNHAM C.E. Comparison of Babcock and ether extraction methods for determination of fat content of milk: collaborative study. J. Assoc. Off. Anal. Chem. 1988;71:898–914. - PubMed
-
- BLAKE M.J., ABDEL-RAHMAN S.M., KEARNS G.L.Effect of diet on caffeine elimination in healthy infants Clin. Pharmacol. Ther. 20047550[abstract]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

