Amadori adducts activate nuclear factor-kappaB-related proinflammatory genes in cultured human peritoneal mesothelial cells
- PMID: 15997235
- PMCID: PMC1576262
- DOI: 10.1038/sj.bjp.0706309
Amadori adducts activate nuclear factor-kappaB-related proinflammatory genes in cultured human peritoneal mesothelial cells
Abstract
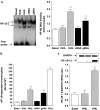
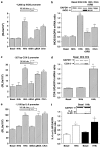
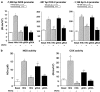
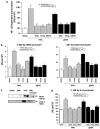
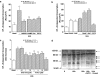
Diabetes mellitus leads to a high incidence of several so-called complications, sharing similar pathophysiological features in several territories. Previous reports points at early nonenzymatic glycosylation products (Amadori adducts) as mediators of diabetic vascular complications. In the present study, we analysed a possible role for Amadori adducts as stimulators of proinflammatory pathways in human peritoneal mesothelial cells (HPMCs). Cultured HPMCs isolated from 13 different patients (mean age 38.7+/-16 years) were exposed to different Amadori adducts, that is, highly glycated haemoglobin (10 nM) and glycated bovine serum albumin (0.25 mg ml(-1)), as well as to their respective low glycosylation controls. Amadori adducts, but not their respective controls, elicited a marked increase of NF-kappaB activation, as determined by electromobility shift assays and transient transfection experiments. Additionally, Amadori adducts significantly increased the production of NF-kappaB-related proinflammatory molecules, including cytokines, such as TNF-alpha, IL-1beta or IL-6, and enzymes, such as cyclooxygenase-2 and inducible nitric oxide (NO) synthase, this latter leading to the release of NO by HPMCs. The effects of Amadori adducts were mediated by different reactive oxygen and nitrosative species (e.g. superoxide anions, hydroxyl radicals, and peroxynitrite), as they were blunted by coincubation with the appropriate scavengers. Furthermore, NO generated upon exposure to Amadori adducts further stimulated NF-kappaB activation, either directly or after combination with superoxide anions to form peroxynitrite. We conclude that Amadori adducts can favour peritoneal inflammation by exacerbating changes in NO synthesis pathway and triggering NF-kappaB-related proinflammatory signals in human mesothelial cells.
Figures
References
-
- AMORE A., CIRINA P., MITOLA S., PERUZZI L., GIANOGLIO B., RABBONE I., SACCHETTI C., CERUTTI F., GRILLO C., COPPO R. Nonenzymatically glycated albumin (Amadori adducts) enhances nitric oxide synthase activity and gene expression in endothelial cells. Kidney Int. 1997;51:27–35. - PubMed
-
- ANGULO J., SÁNCHEZ-FERRER C.F., PEIRÓ C., MARÍN J., RODRÍGUEZ-MAÑAS L. Impairment of endothelium-dependent relaxations by increasing percentages of glycated human haemoglobin. Hypertension. 1996;28:583–592. - PubMed
-
- BERGHE W.V., PLAISANCE S., BOONE E., DE BOSSCHER K., SCHIMITZ M.L., FIERS W., HAEGEMAN G. P38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathway are regulated for nuclear-factor κB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998;273:3285–3290. - PubMed
-
- BODEGA F., ZACCHI L., AGOSTINI E. Albumin transcytosis in mesothelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L3–L11. - PubMed
-
- BOULANGER E., WAUTIER M.P., WAUTIER J.C., BOVAL B., PANIS Y., WERNERT N., DANZE P.M., DEQUIEDT P. AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int. 2002;61:148–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

