Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain
- PMID: 15997239
- PMCID: PMC1576258
- DOI: 10.1038/sj.bjp.0706301
Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain
Abstract
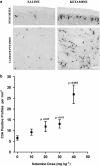
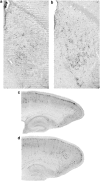
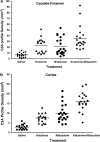
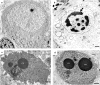
Recently, it was reported that anesthetizing infant rats for 6 h with a combination of anesthetic drugs (midazolam, nitrous oxide, isoflurane) caused widespread apoptotic neurodegeneration in the developing brain, followed by lifelong cognitive deficits. It has also been reported that ketamine triggers neuroapoptosis in the infant rat brain if administered repeatedly over a period of 9 h. The question arises whether less extreme exposure to anesthetic drugs can also trigger neuroapoptosis in the developing brain. To address this question we administered ketamine, midazolam or ketamine plus midazolam subcutaneously at various doses to infant mice and evaluated the rate of neuroapoptosis in various brain regions following either saline or these various drug treatments. Each drug was administered as a single one-time injection in a dose range that would be considered subanesthetic, and the brains were evaluated by unbiased stereology methods 5 h following drug treatment. Neuroapoptosis was detected by immunohistochemical staining for activated caspase-3. It was found that either ketamine or midazolam caused a dose-dependent, statistically significant increase in the rate of neuroapoptosis, and the two drugs combined caused a greater increase than either drug alone. The apoptotic nature of the neurodegenerative reaction was confirmed by electron microscopy. We conclude that relatively mild exposure to ketamine, midazolam or a combination of these drugs can trigger apoptotic neurodegeneration in the developing mouse brain.
Figures

References
-
- BEN-SHLOMO I., ROSENBAUM A., HADASH O., KATZ Y. Intravenous midazolam significantly enhances the lethal effect of thiopental but not that of ketamine in mice. Pharmacol. Res. 2001;44:509–512. - PubMed
-
- CLARREN S.K., ALVORD A.C., SUMI S.M., STREISSGUTH A.P., SMITH D.W. Brain malformations related to prenatal exposure to ethanol. J. Pediatr. 1978;92:64–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

