The timing of TCR alpha expression critically influences T cell development and selection
- PMID: 15998791
- PMCID: PMC2212895
- DOI: 10.1084/jem.20050359
The timing of TCR alpha expression critically influences T cell development and selection
Abstract
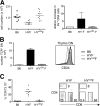
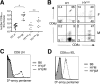
Sequential rearrangement of the T cell receptor for antigen (TCR) beta and alpha chains is a hallmark of thymocyte development. This temporal control is lost in TCR transgenics because the alpha chain is expressed prematurely at the CD4- CD8- double negative (DN) stage. To test the importance of this, we expressed the HY alpha chain at the physiological CD4+ CD8+ double positive (DP) stage. The reduced DP and increased DN cellularity typically seen in TCR transgenics was not observed when the alpha chain was expressed at the appropriate stage. Surprisingly, antigen-driven selection events were also altered. In male mice, thymocyte deletion now occurred at the single positive or medullary stage. In addition, no expansion of CD8 alpha alpha intestinal intraepithelial lymphocytes (IELs) was observed, despite the fact that HY transgenics have been used to model IEL development. Collectively, these data establish the importance of proper timing of TCR expression in thymic development and selection and emphasize the need to use models that most accurately reflect the physiologic process.
Figures




References
-
- Starr, T.K., S.C. Jameson, and K.A. Hogquist. 2003. Positive and negative selection of T cells. Annu. Rev. Immunol. 21:139–176. - PubMed
-
- Trop, S., M. Rhodes, D.L. Wiest, P. Hugo, and J.C. Zuniga-Pflucker. 2000. Competitive displacement of pT alpha by TCR-alpha during TCR assembly prevents surface coexpression of pre-TCR and alpha beta TCR. J. Immunol. 165:5566–5572. - PubMed
-
- Bruno, L., H.J. Fehling, and H. von Boehmer. 1996. The alpha beta T cell receptor can replace the gamma delta receptor in the development of gamma delta lineage cells. Immunity. 5:343–352. - PubMed
-
- Fritsch, M., A. Andersson, K. Petersson, and F. Ivars. 1998. A TCR alpha chain transgene induces maturation of CD4- CD8- alpha beta+ T cells from gamma delta T cell precursors. Eur. J. Immunol. 28:828–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

