Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness
- PMID: 16000375
- PMCID: PMC1196340
- DOI: 10.1091/mbc.e05-02-0131
Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness
Abstract
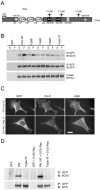
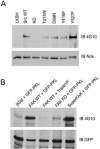
The ArfGAP paxillin kinase linker (PKL)/G protein-coupled receptor kinase-interacting protein (GIT)2 has been implicated in regulating cell spreading and motility through its transient recruitment of the p21-activated kinase (PAK) to focal adhesions. The Nck-PAK-PIX-PKL protein complex is recruited to focal adhesions by paxillin upon integrin engagement and Rac activation. In this report, we identify tyrosine-phosphorylated PKL as a protein that associates with the SH3-SH2 adaptor Nck, in a Src-dependent manner, after cell adhesion to fibronectin. Both cell adhesion and Rac activation stimulated PKL tyrosine phosphorylation. PKL is phosphorylated on tyrosine residues 286/392/592 by Src and/or FAK and these sites are required for PKL localization to focal adhesions and for paxillin binding. The absence of either FAK or Src-family kinases prevents PKL phosphorylation and suppresses localization of PKL but not GIT1 to focal adhesions after Rac activation. Expression of an activated FAK mutant in the absence of Src-family kinases partially restores PKL localization, suggesting that Src activation of FAK is required for PKL phosphorylation and localization. Overexpression of the nonphosphorylated GFP-PKL Triple YF mutant stimulates cell spreading and protrusiveness, similar to overexpression of a paxillin mutant that does not bind PKL, suggesting that failure to recruit PKL to focal adhesions interferes with normal cell spreading and motility.
Figures
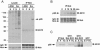


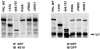



References
-
- Bagrodia, S., Bailey, D., Lenard, Z., Hart, M., Guan, J. L., Premont, R. T., Taylor, S. J., and Cerione, R. A. (1999). A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem. 274, 22393-22400. - PubMed
-
- Bagrodia, S., Taylor, S. J., Jordon, K. A., Van Aelst, L., and Cerione, R. A. (1998). A novel regulator of p21-activated kinases. J. Biol. Chem. 273, 23633-23636. - PubMed
-
- Bladt, F., Aippersbach, E., Gelkop, S., Strasser, G. A., Nash, P., Tafuri, A., Gertler, F. B., and Pawson, T. (2003). The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol. Cell. Biol. 23, 4586-4597. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

