Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons
- PMID: 16000622
- PMCID: PMC6725283
- DOI: 10.1523/JNEUROSCI.1405-05.2005
Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons
Abstract
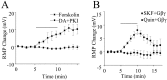
Layer I of the neocortex comprises axonal processes from widespread regions of the brain and a unique population of GABAergic interneurons. Dopamine is known to directly depolarize layer I interneurons, but the underlying mechanism is unclear. Using whole-cell recording techniques in neocortical brain slices, we have examined how dopamine increases excitability of layer I interneurons in postnatal day 7-11 rats. Dopamine (30 microm) caused a 10 mV depolarization of layer I neurons. Paradoxically, neither the D1-like receptor agonist 6-chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide (SKF81297) (1-10 microm) nor the D2-like agonist quinpirole (10 microm) produced a significant depolarization. Depolarization was observed when SKF81297 and quinpirole were coapplied. When G-protein betagamma subunits were included in the recording pipette, D1 but not D2 agonists depolarized layer I neurons. Bath application of 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride, a specific blocker of inwardly rectifying hyperpolarization-activated current (Ih) channels, hyperpolarized the neurons and occluded the action of dopamine. Voltage-clamp analysis demonstrated that dopamine increased the amplitude and shifted the voltage dependence of activation of Ih. These results indicate that Ih contributes to the resting potential of layer I interneurons and is subject to modulation by dopamine.
Figures





References
-
- Berger B (1992) Dopaminergic innervation of the frontal cerebral cortex: evolutionary trends and functional implications. In: Advances in neurology (Chauvel P, Delgado-Escueta AV, Haalgren E, Bancaud J, eds), pp 525-544. New York: Raven. - PubMed
-
- Bernardi G, Cherubini E, Marciani MG, Mercuri N, Stanzione P (1982) Responses of intracellularly recorded cortical neurons to the iontophoretic application of dopamine. Brain Res 245: 267-274. - PubMed
-
- Bradford R, Parnavelas JG, Lieberman AR (1978) Neurons in layer I of the developing occipital cortex of the rat. J Comp Neurol 176: 121-132. - PubMed
-
- Christophe E, Roebuck A, Staiger JF, Lavery DJ, Charpak S, Audinat E (2002) Two types of nicotinic receptors mediate an excitation of neocortical layer I interneurons. J Neurophysiol 88: 1318-1327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
