Suppression of damping-off disease in host plants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soilborne Peronosporomycetes
- PMID: 16000790
- PMCID: PMC1169021
- DOI: 10.1128/AEM.71.7.3786-3796.2005
Suppression of damping-off disease in host plants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soilborne Peronosporomycetes
Abstract
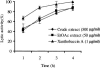
We previously demonstrated that xanthobaccin A from the rhizoplane bacterium Lysobacter sp. strain SB-K88 suppresses damping-off disease caused by Pythium sp. in sugar beet. In this study we focused on modes of Lysobacter sp. strain SB-K88 root colonization and antibiosis of the bacterium against Aphanomyces cochlioides, a pathogen of damping-off disease. Scanning electron microscopic analysis of 2-week-old sugar beet seedlings from seeds previously inoculated with SB-K88 revealed dense colonization on the root surfaces and a characteristic perpendicular pattern of Lysobacter colonization possibly generated via development of polar, brush-like fimbriae. In colonized regions a semitransparent film apparently enveloping the root and microcolonies were observed on the root surface. This Lysobacter strain also efficiently colonized the roots of several plants, including spinach, tomato, Arabidopsis thaliana, and Amaranthus gangeticus. Plants grown from both sugar beet and spinach seeds that were previously treated with Lysobacter sp. strain SB-K88 displayed significant resistance to the damping-off disease triggered by A. cochlioides. Interestingly, zoospores of A. cochlioides became immotile within 1 min after exposure to a SB-K88 cell suspension, a cell-free supernatant of SB-K88, or pure xanthobaccin A (MIC, 0.01 microg/ml). In all cases, lysis followed within 30 min in the presence of the inhibiting factor(s). Our data indicate that Lysobacter sp. strain SB-K88 has a direct inhibitory effect on A. cochlioides, suppressing damping-off disease. Furthermore, this inhibitory effect of Lysobacter sp. strain SB-K88 is likely due to a combination of antibiosis and characteristic biofilm formation at the rhizoplane of the host plant.
Figures








References
-
- Bonner, D. P., J. O'Sullivan, S. K. Tanaka, J. M. Clark, and R. R. Whitney. 1988. Lysobactin, a novel antibacterial agent produced by Lysobacter sp. II. Biological properties. J. Antibiot. 41:1745-1751. - PubMed
-
- Chin-A-Woeng, T. F. C., W. D. Priester, A. J. V. D. Bij, and B. J. J. Lugtenburg. 1997. Description of the colonization of a gnotobiotic tomato rhizosphere by Pseudomonas fluorescens biocontrol strain WCS365, using scanning electron microscopy. Mol. Plant-Microbe Interact. 10:79-86.
-
- Christensen, P., and F. D. Cook. 1978. Lysobacter, a new genus of non-fruiting, gliding bacteria with high base ratio. Int. J. Syst. Bacteriol. 28:367-393.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

