Susceptibility of biofilms to Bdellovibrio bacteriovorus attack
- PMID: 16000819
- PMCID: PMC1169041
- DOI: 10.1128/AEM.71.7.4044-4051.2005
Susceptibility of biofilms to Bdellovibrio bacteriovorus attack
Abstract
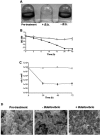
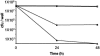
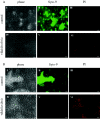
Biofilms are communities of microorganisms attached to a surface, and the growth of these surface attached communities is thought to provide microorganisms with protection against a range of biotic and abiotic agents. The capability of the gram-negative predatory bacterium Bdellovibrio bacteriovorus to control and reduce an existing Escherichia coli biofilm was evaluated in a static assay. A reduction in biofilm biomass was observed as early as 3 h after exposure to the predator, and an 87% reduction in crystal violet staining corresponding to a 4-log reduction in biofilm cell viability was seen after a 24-h exposure period. We observed that an initial titer of Bdellovibrio as low as 10(2) PFU/well or an exposure to the predator as short as 30 min is sufficient to reduce a preformed biofilm. The ability of B. bacteriovorus to reduce an existing biofilm was confirmed by scanning electron microscopy. The reduction in biofilm biomass obtained after the first 24 h of exposure to the predator remained unchanged even after longer exposure periods and reinoculation of the samples with fresh Bdellovibrio; however, no genetically stable resistant population of the host bacteria could be detected. Our data suggest that growth in a biofilm does not prevent predation by Bdellovibrio but allows a level of survival from attack greater than that observed for planktonic cells. In flow cell experiments B. bacteriovorus was able to decrease the biomass of both E. coli and Pseudomonas fluorescens biofilms as determined by phase-contrast and epifluorescence microscopy.
Figures
References
-
- Chen, X., and P. S. Stewart. 2000. Biofilm removal caused by chemical treatments. Water Res. 34:4229-4233.
-
- Christensen, B. B., C. Sternberg, J. B. Andersen, R. J. Palmer, Jr., A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. - PubMed
-
- Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

