The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells
- PMID: 16001084
- PMCID: PMC1176464
- DOI: 10.1038/sj.emboj.7600738
The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells
Abstract
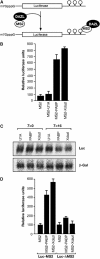
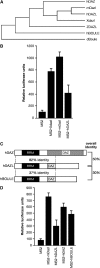
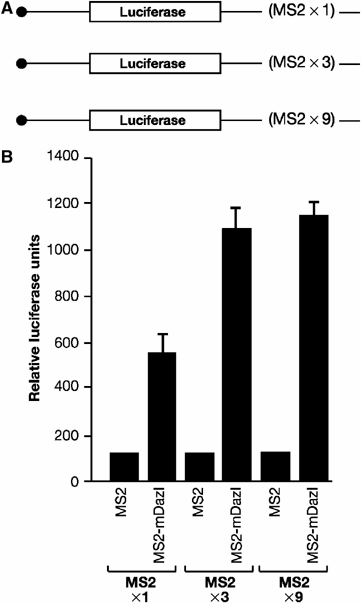
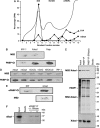
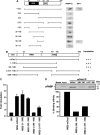
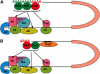
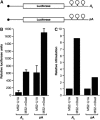
DAZL proteins are germ-cell-specific RNA-binding proteins essential for gametogenesis. The precise molecular role of these proteins in germ-cell development remains enigmatic; however, they appear to function in the cytoplasm. In order to directly address the function of vertebrate DAZL proteins, we have used Xenopus laevis oocytes as a model system. Here we demonstrate that members of this family, including Xdazl, mouse Dazl, human DAZL, human DAZ and human BOULE, have the ability to stimulate translation and function at the level of translation initiation. We show that DAZL proteins interact with poly(A)-binding proteins (PABPs), which are critical for the initiation of translation. Mapping and tethered function experiments suggest that these interactions are physiologically important. This leads to an attractive hypothesis whereby DAZL proteins activate translationally silent mRNAs during germ cell development through the direct recruitment of PABPs.
Figures
References
-
- Cheng MH, Maines JZ, Wasserman SA (1998) Biphasic subcellular localization of the DAZL-related protein boule in Drosophila spermatogenesis. Dev Biol 204: 567–576 - PubMed
-
- Cosson B, Couturier A, Le Guellec R, Moreau J, Chabelskaya S, Zhouravleva G, Philippe M (2002) Characterization of the poly(A) binding proteins expressed during oogenesis and early development of Xenopus laevis. Biol Cell 94: 217–231 - PubMed
-
- de Kretser DM (1997) Male infertility. Lancet 349: 787–790 - PubMed
-
- Eberhart CG, Maines JZ, Wasserman SA (1996) Meiotic cell cycle requirement for a fly homologue of human Deleted in Azoospermia. Nature 381: 783–785 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

