A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics
- PMID: 16002427
- PMCID: PMC1895336
- DOI: 10.1182/blood-2005-03-1288
A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics
Abstract
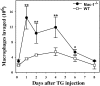
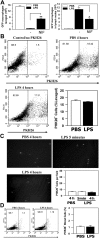
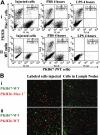
In response to injury, monocytes migrate to the site of inflammation, where they differentiate into macrophages and participate in various biologic processes. However, their fate during the resolution of acute inflammation is not fully understood. Here, we show that inflammatory macrophages do not die locally by apoptosis; rather, they migrate across the peritoneal mesothelium to the lymphatics, through which they further migrate to the lymph nodes and to the blood circulation. Macrophage efflux is enhanced considerably on cell activation, and such accelerated macrophage migration is dependent specifically on integrin Mac-1, and can be blocked by addition of its antagonist. Thus, genetic inactivation of Mac-1 in mice inhibits the accelerated macrophage efflux from the inflammatory site to the lymphatics, but it does not compromise the accumulation of blood monocytes into the inflammatory site. Together, our study demonstrates that Mac-1 is involved specifically in the efflux of activated macrophages to the lymphatics, suggesting that Mac-1 may play an important role in the removal of local inflammatory macrophages and in their subsequent migration to the lymph nodes, a process that is critical to the development of the adaptive immunity.
Figures



References
-
- Furie MB, Tancinco MCA, Smith CW. Monoclonal antibodies to leukocyte integrins CD11a/CD18 and CD11b/CD18 or intercellular adhesion molecule-1 inhibit chemoattractant-stimulated neutrophil transendothelial migration in vitro. Blood. 1991;78: 2089-2097. - PubMed
-
- Andrew DP, Spellberg JP, Takimoto H, et al. Transendothelial migration and trafficking of leukocytes in LFA-1-deficient mice. Eur J Immunol. 1998;28: 1959-1969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

