SyrB2 in syringomycin E biosynthesis is a nonheme FeII alpha-ketoglutarate- and O2-dependent halogenase
- PMID: 16002467
- PMCID: PMC1177402
- DOI: 10.1073/pnas.0504412102
SyrB2 in syringomycin E biosynthesis is a nonheme FeII alpha-ketoglutarate- and O2-dependent halogenase
Abstract
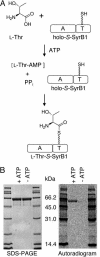
The nine-residue lipodepsipeptide syringomycin E, elaborated as a phytotoxin by Pseudomonas syringae pv. syringae B301D contains a 4-Cl-L-Thr-9 moiety where failure to chlorinate results in a 3-fold drop in biological activity. The proteins SyrB1 and SyrB2 encoded by the biosynthetic cluster are shown to act as a substrate and enzyme pair for SyrB2-mediated chlorination of the aminoacyl-S-enzyme L-Thr-S-SyrB1. SyrB2 is a member of the nonheme Fe(II) alpha-ketoglutarate-dependent enzyme superfamily, and requires O2 and alpha-ketoglutarate as well as chloride ion to carry out monochlorination of the -CH3 group of L-Thr-S-SyrB1. Chlorination of L-Thr-S-SyrB1 was validated by thioesterase-mediated release of L-Thr and 4-Cl-L-Thr, N-derivatization as fluorescent isoindoles, and HPLC separation compared with authentic standards. Incubations with L-[14C]Thr and [36Cl-] as well as MS of the released products further validated identification. Enzymatic oxidative halogenation is a previously uncharacterized reaction type for nonheme Fe(II) enzymes and may be the general mode for biosynthetic halogenation of aliphatic carbons of natural products.
Figures




References
-
- Gribble, G. W. (2004) J. Chem. Educ. 81, 1441-1449.
-
- Kahne, D., Leimkuhler, C., Lu, W. & Walsh, C. T. (2005) Chem. Rev. 105, 425-448. - PubMed
-
- Ryan, M. J., Lotvin, J. A., Strathy, N. & Fantini, S. E. (1996) U.S. Patent 5,589,385.
-
- Pojer, F., Li, S. M. & Heide, L. (2002) Microbiology 148, 3901-3911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

