Replication and packaging of Norwalk virus RNA in cultured mammalian cells
- PMID: 16002473
- PMCID: PMC1177355
- DOI: 10.1073/pnas.0408529102
Replication and packaging of Norwalk virus RNA in cultured mammalian cells
Abstract
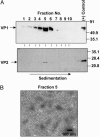
Human noroviruses, the most common cause of nonbacterial gastroenteritis, are characterized by high infectivity rate, low infectious dose, and unusually high stability outside the host. However, human norovirus research is hindered by the lack of a cell culture system and a small animal model of infection. Norwalk virus (NV) is the prototype strain of human noroviruses. We report here replication of NV viral RNA and its packaging into virus particles in mammalian cells by intracellular expression of native forms of NV viral RNA devoid of extraneous nucleotide sequences derived from the expression vector by the use of replication-deficient vaccinia virus MVA encoding the bacteriophage T7 RNA polymerase (MVA/T7). Expressed genomic RNA was found to replicate; NV subgenomic RNA was transcribed from genomic RNA by use of NV nonstructural proteins expressed from genomic RNA and was subsequently translated into NV capsid protein VP1. Viral genomic RNA was packaged into virus particles generated in mammalian cells. The cesium chloride (CsCl) density gradient profile of virus particles containing genomic RNA was similar to that of NV purified from stool. These observations indicate that the NV cDNA constructed here is a biologically infectious clone, and that mammalian cells have the ability to replicate NV genomic RNA. This work establishes a mammalian cell-based system for analysis of human norovirus replication and, thus, makes it feasible to investigate antiviral agents in mammalian cells.
Figures


References
-
- Billgren, M., Christenson, B., Hedlund, K. O. & Vinje, J. (2002) J. Infect. 44, 26-32. - PubMed
-
- Fankhauser, R. L., Noel, J. S., Monroe, S. S., Ando, T. & Glass, R. I. (1998) J. Infect. Dis. 178, 1571-1578. - PubMed
-
- Jiang, X., Wang, M., Wang, K. & Estes, M. K. (1993) Virology 195, 51-61. - PubMed
-
- Green, K. Y., Chanock, R. M. & Kapikian, A. Z. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott Williams & Wilkins, Philadephia), pp. 841-874.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

