Phylogenetic analysis of the phytochrome superfamily reveals distinct microbial subfamilies of photoreceptors
- PMID: 16004604
- PMCID: PMC1317669
- DOI: 10.1042/BJ20050826
Phylogenetic analysis of the phytochrome superfamily reveals distinct microbial subfamilies of photoreceptors
Abstract
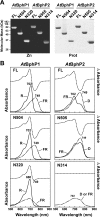
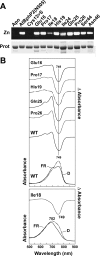
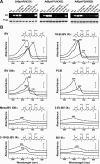
Phys (phytochromes) are a superfamily of photochromic photoreceptors that employ a bilin-type chromophore to sense red and far-red light. Although originally thought to be restricted to plants, accumulating genetic and genomic analyses now indicate that they are also prevalent among micro-organisms. By a combination of phylogenetic and biochemical studies, we have expanded the Phy superfamily and organized its members into distinct functional clades which include the phys (plant Phys), BphPs (bacteriophytochromes), Cphs (cyanobacterial Phys), Fphs (fungal Phys) and a collection of Phy-like sequences. All contain a signature GAF (cGMP phosphodiesterase/adenylate cyclase/FhlA) domain, which houses the bilin lyase activity. A PHY domain (uppercase letters are used to denote the PHY domain specifically), which helps stabilize the Pfr form (far-red-light-absorbing form of Phy), is downstream of the GAF region in all but the Phy-like sequences. The phy, Cph, BphP and Fph families also include a PLD [N-terminal PAS (Per/Arnt/Sim)-like domain] upstream of the GAF domain. Site-directed mutagenesis of conserved residues within the GAF and PLD motifs supports their importance in chromophore binding and/or spectral activity. In agreement with Lamparter, Carrascal, Michael, Martinez, Rottwinkel and Abian [(2004) Biochemistry 43, 3659-3669], a conserved cysteine within the PLD of several BphPs was found to be necessary for binding the chromophore via the C-3 vinyl side chain on the bilin A ring. Phy-type sequences were also discovered in the actinobacterium Kineococcus radiotolerans and collections of microorganisms obtained from marine and extremely acidic environments, thus expanding further the range of these photoreceptors. Based on their organization and distribution, the evolution of the Phy superfamily into distinct photoreceptor types is proposed.
Figures




References
-
- Briggs W. R., Spudich J. L. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co; 2005. Handbook of Photosensory Systems.
-
- Quail P. H. Phytochrome photosensory signalling networks. Nat. Rev. Mol. Cell Biol. 2002;3:85–93. - PubMed
-
- Smith H. Phytochromes and light signal perception by plants – an emerging synthesis. Nature (London) 2000;407:585–591. - PubMed
-
- Vierstra R. D. Cyanophytochromes, bacteriophytochromes, and plant phytochromes: light-regulated kinases related to bacterial two-component regulators. In: Inouya M., Dutta R., editors. Histidine Kinases in Signal Transduction. New York: Academic Press; 2002. pp. 273–295.
-
- Bhoo S. H., Davis S. J., Walker J., Karniol B., Vierstra R. D. Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore. Nature (London) 2001;414:776–779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

