NMDA-induced potentiation of mGluR5 is mediated by activation of protein phosphatase 2B/calcineurin
- PMID: 16005030
- PMCID: PMC3799794
- DOI: 10.1016/j.neuropharm.2005.05.005
NMDA-induced potentiation of mGluR5 is mediated by activation of protein phosphatase 2B/calcineurin
Abstract
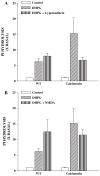
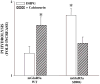
Previous reports have shown that activation of N-methyl-D-aspartate (NMDA) receptors potentiates responses to activation of the group I metabotropic glutamate receptor mGluR5 by reversing PKC-mediated desensitization of this receptor. NMDA-induced reversal of mGluR5 desensitization is dependent on activation of protein phosphatases. However, the specific protein phosphatase involved and the precise mechanism by which NMDA receptor activation reduces mGluR desensitization are not known. We have performed a series of molecular, biochemical, and genetic studies to show that NMDA-induced regulation of mGluR5 is dependent on activation of calcium-dependent protein phosphatase 2B/calcineurin (PP2B/CaN). Furthermore, we report that purified calcineurin directly dephosphorylates the C-terminal tail of mGluR5 at sites that are phosphorylated by PKC. Finally, immunoprecipitation and GST fusion protein pull-down experiments reveal that calcineurin interacts with mGluR5, suggesting that these proteins could be colocalized in a signaling complex. Taken together with previous studies, these data suggest that activation of NMDA receptors leads to activation of calcineurin and that calcineurin modulates mGluR5 function by directly dephosphorylating mGluR5 at PKC sites that are involved in desensitization of this receptor.
Figures




References
-
- Alagarsamy S, Marino MJ, Rouse ST, Gereau RW, Heinemann SF, Conn PJ. Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci. 1999a;2:234–240. - PubMed
-
- Alagarsamy S, Rouse ST, Gereau RW, Heinemann SF, Smith Y, Conn PJ. Activation of N-methyl-D-aspartate receptors reverses desensitization of metabotropic glutamate receptor, mGluR5, in native and recombinant systems. Ann N Y Acad Sci. 1999b;868:526–530. - PubMed
-
- Aniksztejn L, Bregestovski P, Ben Ari Y. Selective activation of quisqualate metabotropic receptor potentiates NMDA but not AMPA responses. Eur J Pharmacol. 1991;205:327–328. - PubMed
-
- Bleakman D, Rusin KI, Chard PS, Glaum SR, Miller RJ. Metabotropic glutamate receptors potentiate ionotropic glutamate responses in the rat dorsal horn. Mol Pharmacol. 1992;42:192–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

