Space- and time-resolved spectrophotometry in microsystems
- PMID: 16006503
- PMCID: PMC1177425
- DOI: 10.1073/pnas.0504712102
Space- and time-resolved spectrophotometry in microsystems
Abstract
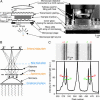
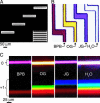
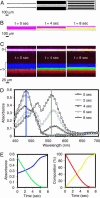
This work describes a simple optical method for obtaining, in a single still-capture image, the continuous absorbance spectra of samples at multiple locations of microsystems. This technique uses an unmodified bright-field microscope, an array of microlenses, and a diffraction grating to disperse the light transmitted by samples of 10- to 500-microm dimensions. By analyzing in a single image the first-order diffracted light, it is possible to collect the full and continuous absorbance spectra of samples at multiple locations (to a spatial resolution of approximately 8 microm) in microwells and microchannels to examine dynamic chemical events (to a time resolution of <10 ms). This article also discusses the optical basis of this method. The simultaneous resolution of wavelength, time, and space at a scale <10 microm provides additional capabilities for chemical and biological analysis.
Figures

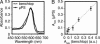
Similar articles
-
Microscopic spectral imaging using a video camera.J Microsc. 1986 Jan;141(Pt 1):31-9. doi: 10.1111/j.1365-2818.1986.tb02698.x. J Microsc. 1986. PMID: 3959067
-
Optical time-of-flight and absorbance imaging of biologic media.Science. 1993 Mar 5;259(5100):1463-6. doi: 10.1126/science.8451643. Science. 1993. PMID: 8451643
-
New multichannel kinetic spectrophotometer-fluorimeter with pulsed measuring beam for photosynthesis research.Photosynth Res. 2006 Jun;88(3):351-6. doi: 10.1007/s11120-006-9071-y. Epub 2006 Jun 9. Photosynth Res. 2006. PMID: 16763876
-
Optical spectroscopy. Principles and instrumentation.Methods Mol Biol. 1994;22:151-71. doi: 10.1385/0-89603-232-9:151. Methods Mol Biol. 1994. PMID: 8312991 Review. No abstract available.
-
[Dynamic light scattering].Tanpakushitsu Kakusan Koso. 2004 Aug;49(11 Suppl):1676-80. Tanpakushitsu Kakusan Koso. 2004. PMID: 15376999 Review. Japanese. No abstract available.
Cited by
-
Tunable visual color filter using microfluidic grating.Biomicrofluidics. 2010 Dec 30;4(4):43013. doi: 10.1063/1.3491469. Biomicrofluidics. 2010. PMID: 21267090 Free PMC article.
-
Self-loading lithographically structured microcontainers: 3D patterned, mobile microwells.Lab Chip. 2008 Oct;8(10):1621-4. doi: 10.1039/b809098j. Epub 2008 Sep 1. Lab Chip. 2008. PMID: 18813382 Free PMC article.
-
Reactions in droplets in microfluidic channels.Angew Chem Int Ed Engl. 2006 Nov 13;45(44):7336-56. doi: 10.1002/anie.200601554. Angew Chem Int Ed Engl. 2006. PMID: 17086584 Free PMC article. Review.
References
-
- Vilkner, T., Janasek, D. & Manz, A. (2004) Anal. Chem. 76, 3373-3386. - PubMed
-
- Losey, M. W., Schmidt, M. A. & Jensen, K. F. (2001) Ind. Eng. Chem. Res. 40, 2555-2562.
-
- Dunn, D. A. & Feygin, I. (2000) Drug Discovery Today 5, S84-S91. - PubMed
-
- Huang, L. R., Cox, E. C., Austin, R. H. & Sturm, J. C. (2004) Science 304, 987-990. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

