A reversible molecular valve
- PMID: 16006520
- PMCID: PMC1174922
- DOI: 10.1073/pnas.0504109102
A reversible molecular valve
Abstract
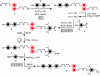
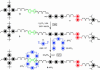
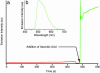
In everyday life, a macroscopic valve is a device with a movable control element that regulates the flow of gases or liquids by blocking and opening passageways. Construction of such a device on the nanoscale level requires (i) suitably proportioned movable control elements, (ii) a method for operating them on demand, and (iii) appropriately sized passageways. These three conditions can be fulfilled by attaching organic, mechanically interlocked, linear motor molecules that can be operated under chemical, electrical, or optical stimuli to stable inorganic porous frameworks (i.e., by self-assembling organic machinery on top of an inorganic chassis). In this article, we demonstrate a reversibly operating nanovalve that can be turned on and off by redox chemistry. It traps and releases molecules from a maze of nanoscopic passageways in silica by controlling the operation of redox-activated bistable [2]rotaxane molecules tethered to the openings of nanopores leading out of a nanoscale reservoir.
Figures
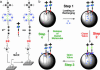
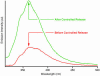
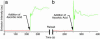
References
-
- Hernandez, R., Tseng, H.-R., Wong, J. W., Stoddart, J. F. & Zink, J. I. (2004) J. Am. Chem. Soc. 126, 3370-3371. - PubMed
-
- Liu, N., Dunphy, D. R., Atanassov, P., Bunge, S. D., Chen, Z., Lopez, G. P., Boyle, T. J. & Brinker, J. C. (2004) Nano Lett. 4, 551-554.
-
- Mal, N. K., Fujiwara, M. & Tanaka, Y. (2003) Nature 421, 350-353. - PubMed
-
- Mal, N. K., Fujiwara, M., Tanaka, Y., Taguchi, T. & Matsukata, M. (2003) Chem. Mater. 15, 3385-3394.
-
- Lai, C.-Y., Trewyn, B. G., Jeftinija, D. M., Jeftinija, K., Xu S., Jeftinija, S. & Lin, V. S.-Y. (2003) J. Am. Chem. Soc. 125, 4451-4459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

