Adhesion signaling by a novel mitotic substrate of src kinases
- PMID: 16007225
- PMCID: PMC3023961
- DOI: 10.1038/sj.onc.1208582
Adhesion signaling by a novel mitotic substrate of src kinases
Abstract
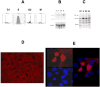
Src kinases are activated and relocalize to the cytoplasm during mitosis, but their mitotic function has remained elusive. We describe here a novel mitotic substrate of src kinases. Trask (transmembrane and associated with src kinases) is a 140 kDa type I transmembrane glycoprotein unrelated to currently known protein families. Src kinases phosphorylate Trask in vitro and mediate its mitotic hyperphosphorylation in vivo. Trask associates with both yes and src, is localized to the cell membrane during interphase, and undergoes cytoplasmic relocalization during mitosis. Overexpression of Trask leads to cell rounding and a loss of adhesion phenotype. Consistent with a function in cell adhesion, Trask interacts with a number of adhesion and matrix proteins including cadherins, syndecans, and the membrane-type serine protease 1 (MT-SP1), and is proteolytically cleaved by MT-SP1. Trask is unique among cell adhesion molecules in that it is under cell cycle regulation and thus links src kinases with the mitotic regulation of cell adhesion. This suggests a potential pathway by which hyperactive src kinases in tumors can deregulate adhesion signaling and mediate the metastatic phenotype.
Figures





References
-
- Bagrodia S, Chackalaparampil I, Kmiecik TE, Shalloway D. Nature. 1991;349:172–175. - PubMed
-
- Bagrodia S, laudano AP, Shalloway D. J Biol Chem. 1994;269:10247–10251. - PubMed
-
- Bhatt AS, Takeuchi T, Ylstra B, Ginzinger D, Albertson D, Shuman MA, Craik CS. Biological Chemistry. 2003;384:257–266. - PubMed
-
- Boyer B, Bourgeois Y, Poupon MF. Oncogene. 2002;21:2347–2356. - PubMed
-
- Brown TA, Yang TM, Zaitsevskaia T, Xia Y, Dunn CA, Sigle RO, Knudsen B, Carter WG. J Biol Chem. 2004;279:14772–14783. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

