Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease
- PMID: 16007245
- PMCID: PMC1159152
- DOI: 10.1172/JCI25669
Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease
Abstract
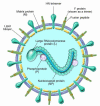
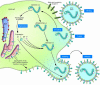
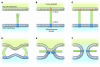
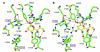
Human parainfluenza viruses cause several serious respiratory diseases in children for which there is no effective prevention or therapy. Parainfluenza viruses initiate infection by binding to cell surface receptors and then, via coordinated action of the 2 viral surface glycoproteins, fuse directly with the cell membrane to release the viral replication machinery into the host cell's cytoplasm. During this process, the receptor-binding molecule must trigger the viral fusion protein to mediate fusion and entry of the virus into a cell. This review explores the binding and entry into cells of parainfluenza virus type 3, focusing on how the receptor-binding molecule triggers the fusion process. There are several steps during the process of binding, triggering, and fusion that are now understood at the molecular level, and each of these steps represents potential targets for interrupting infection.
Figures

References
-
- Collins, P., Chanock, R., and McIntosh, K. 1996. Parainfluenza viruses. In Fields virology. B. Fields et al., editors. Lippincott-Raven Publishers. Philadelphia, Pennsylvania, USA. 1205–1241.
-
- Chanock RM. Control of pediatric viral diseases: past successes and future prospects [review] Pediatr. Res. 1990;27(Suppl.):S39–S43. - PubMed
-
- Groothuis J, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N. Engl. J. Med. 1993;329:1524–1530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

