Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog
- PMID: 16007257
- PMCID: PMC1159140
- DOI: 10.1172/JCI24354
Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog
Abstract
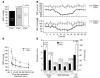
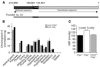
The secretory prohormone chromogranin A (CHGA) is overexpressed in essential hypertension, a complex trait with genetic predisposition, while its catecholamine release-inhibitory fragment catestatin is diminished, and low catestatin predicts augmented adrenergic pressor responses. These findings from studies on humans suggest a mechanism whereby diminished catestatin might increase the risk for hypertension. We generated Chga and humanized mice through transgenic insertion of a human CHGA haplotype in order to probe CHGA and catestatin in vivo. Chga mice displayed extreme phenotypic changes, including: (a) decreased chromaffin granule size and number; (b) elevated BP; (c) loss of diurnal BP variation; (d) increased left ventricular mass and cavity dimensions; (e) decreased adrenal catecholamine, neuropeptide Y (Npy), and ATP contents; (f) increased catecholamine/ATP ratio in the chromaffin granule; and (g) increased plasma catecholamine and Npy levels. Rescue of elevated BP to normalcy was achieved by either exogenous catestatin replacement or humanization of Chga mice. Loss of the physiological "brake" catestatin in Chga mice coupled with dysregulation of transmitter storage and release may act in concert to alter autonomic control of the circulation in vivo, eventuating in hypertension.
Figures


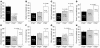


Comment in
-
Chromogranin A: a surprising link between granule biogenesis and hypertension.J Clin Invest. 2005 Jul;115(7):1711-3. doi: 10.1172/JCI25706. J Clin Invest. 2005. PMID: 16007250 Free PMC article. Review.
References
-
- Taupenot L, Harper KL, O’Connor DT. Mechanisms of disease: the chromogranin-secretogranin family. N. Engl. J. Med. 2003;348:1134–1149. - PubMed
-
- Takiyyuddin MA, et al. Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation. 1990;81:185–195. - PubMed
-
- Winkler H, Apps DK, Fischer-Colbrie R. The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience. 1986;18:261–290. - PubMed
-
- Videen JS, Mezger MS, Chang YM, O’Connor DT. Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J. Biol. Chem. 1992;267:3066–3073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

