Phenotypic analysis of trypanothione synthetase knockdown in the African trypanosome
- PMID: 16008527
- PMCID: PMC1276942
- DOI: 10.1042/BJ20050911
Phenotypic analysis of trypanothione synthetase knockdown in the African trypanosome
Abstract
Trypanothione plays a pivotal role in defence against chemical and oxidant stress, thiol redox homoeostasis, ribonucleotide metabolism and drug resistance in parasitic kinetoplastids. In Trypanosoma brucei, trypanothione is synthesized from glutathione and spermidine by a single enzyme, TryS (trypanothione synthetase), with glutathionylspermidine as an intermediate. To examine the physiological roles of trypanothione, tetracycline-inducible RNA interference was used to reduce expression of TRYS. Following induction, TryS protein was reduced >10-fold and growth rate was reduced 2-fold, with concurrent 5-10-fold decreases in glutathionylspermidine and trypanothione and an up to 14-fold increase in free glutathione content. Polyamine levels were not significantly different from non-induced controls, and neither was the intracellular thiol redox potential, indicating that these factors are not responsible for the growth defect. Compensatory changes in other pathway enzymes were associated with prolonged suppression of TryS: an increase in trypanothione reductase and gamma-glutamylcysteine synthetase, and a transient decrease in ornithine decarboxylase. Depleted trypanothione levels were associated with increases in sensitivity to arsenical, antimonial and nitro drugs, implicating trypanothione metabolism in their mode of action. Escape mutants arose after 2 weeks of induction, with all parameters, including growth, returning to normal. Selective inhibitors of TryS are required to fully validate this novel drug target.
Figures
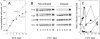
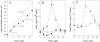
References
-
- World Health Organization. The World Health Report 2002: Reducing risks, promoting healthy life. Geneva: World Health Organization; 2002. Statistical Annex; pp. 169–232.
-
- Fairlamb A. H. Target discovery and validation with special reference to trypanothione. In: Fairlamb A. H., Ridley R. G., Vial H. J., editors. Drugs Against Parasitic Diseases: R&D Methodologies and Issues. Geneva: TDR Publications, World Health Organization; 2003. pp. 107–118.
-
- Fairlamb A. H., Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 1992;46:695–729. - PubMed
-
- Shames S. L., Fairlamb A. H., Cerami A., Walsh C. T. Purification and characterization of trypanothione reductase from Crithidia fasciculata, a newly discovered member of the family of disulphide-containing flavoprotein reductases. Biochemistry. 1986;25:3519–3526. - PubMed
-
- Henderson G. B., Fairlamb A. H., Cerami A. Trypanothione dependent peroxide metabolism in Crithidia fasciculata and Trypanosoma brucei. Mol. Biochem. Parasitol. 1987;24:39–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

