Identification of critical active-site residues in angiotensin-converting enzyme-2 (ACE2) by site-directed mutagenesis
- PMID: 16008552
- PMCID: PMC7164114
- DOI: 10.1111/j.1742-4658.2005.04756.x
Identification of critical active-site residues in angiotensin-converting enzyme-2 (ACE2) by site-directed mutagenesis
Abstract
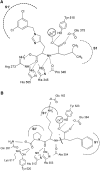
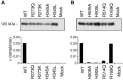
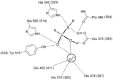
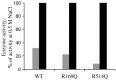
Angiotensin-converting enzyme-2 (ACE2) may play an important role in cardiorenal disease and it has also been implicated as a cellular receptor for the severe acute respiratory syndrome (SARS) virus. The ACE2 active-site model and its crystal structure, which was solved recently, highlighted key differences between ACE2 and its counterpart angiotensin-converting enzyme (ACE), which are responsible for their differing substrate and inhibitor sensitivities. In this study the role of ACE2 active-site residues was explored by site-directed mutagenesis. Arg273 was found to be critical for substrate binding such that its replacement causes enzyme activity to be abolished. Although both His505 and His345 are involved in catalysis, it is His345 and not His505 that acts as the hydrogen bond donor/acceptor in the formation of the tetrahedral peptide intermediate. The difference in chloride sensitivity between ACE2 and ACE was investigated, and the absence of a second chloride-binding site (CL2) in ACE2 confirmed. Thus ACE2 has only one chloride-binding site (CL1) whereas ACE has two sites. This is the first study to address the differences that exist between ACE2 and ACE at the molecular level. The results can be applied to future studies aimed at unravelling the role of ACE2, relative to ACE, in vivo.
Figures
References
-
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G & Turner AJ (2000) A human homolog of angiotensin‐converting enzyme: cloning and functional expression as a captopril‐insensitive carboxypeptidase. J Biol Chem 275, 33238–33243. - PubMed
-
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R et al. (2000) A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87, E1–E9. - PubMed
-
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F et al. (2002) Hydrolysis of biological peptides by human angiotensin‐converting enzyme‐related carboxypeptidase. J Biol Chem 277, 14838–14843. - PubMed
-
- Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira‐dos‐Santos AJ, da Costa J, Zhang L, Pei Y et al. (2002) Angiotensin‐converting enzyme 2 is an essential regulator of heart function. Nature 417, 822–828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

