Effect of ondansetron, a 5-HT3 receptor antagonist, on fatigue in chronic hepatitis C: a randomised, double blind, placebo controlled study
- PMID: 16009690
- PMCID: PMC1774898
- DOI: 10.1136/gut.2004.055251
Effect of ondansetron, a 5-HT3 receptor antagonist, on fatigue in chronic hepatitis C: a randomised, double blind, placebo controlled study
Abstract
Background and aims: There are no available effective therapies for fatigue associated with chronic hepatitis C (CHC). The serotonin antagonist ondansetron has been shown to be effective in the chronic fatigue syndrome. In this randomised, placebo controlled, double blind trial, we investigated the effect of orally administered ondansetron on fatigue in CHC.
Methods: Thirty six patients with CHC were included if fatigue was their predominant symptom and they scored more than 4 on a visual analogue scale (0-10). During the study, fatigue and depression were measured on days 0, 15, 30, and 60 using a validated self report questionnaire (fatigue impact scale and Beck depression inventory). Patients were randomised to receive ondansetron tablets 4 mg twice daily or placebo for one month followed by an additional four weeks of observation.
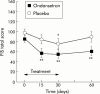
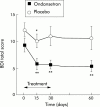
Results: Fatigue score was 85.4 (28.2) and 98.2 (26.9) in the ondansetron and placebo groups, respectively (NS). Ondansetron significantly reduced the fatigue score with more than 30% improvement on day 15 (57.1 (38.9); p<0.01), day 30 (54.5 (37.6); p<0.01), and day 60 (60.8 (37.3); p<0.01) whereas placebo did not. Overall, the reduction in fatigue was significantly higher with ondansetron compared with placebo (ANOVA for repeated measurements) for the whole follow up period (p = 0.03) or for the treatment period only (p = 0.04). Ondansetron also significantly reduced depression scores.
Conclusions: The 5-hydroxytryptamine receptor type 3 antagonist ondansetron had a significant positive effect on fatigue in CHC. These observations support the concept that fatigue involves serotoninergic pathways and may encourage further evaluations of the efficacy of ondansetron on fatigue in chronic liver diseases.
Figures
Comment in
-
5-HT3 receptor antagonists ameliorate fatigue: so much potential, so little knowledge!Gut. 2005 Aug;54(8):1056-7. doi: 10.1136/gut.2004.063545. Gut. 2005. PMID: 16009677 Free PMC article.
References
-
- Kroenke MD, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care. JAMA 1988;260:929–34. - PubMed
-
- Gumber SC, Chopra S. Hepatitis C. A multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med 1995;123:615–20. - PubMed
-
- Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology 1998;27:209–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
